Study on the effect of vanadium source on the electrochemical performance of sodium vanadium phosphate cathode materials for sodium-ion batteries
-
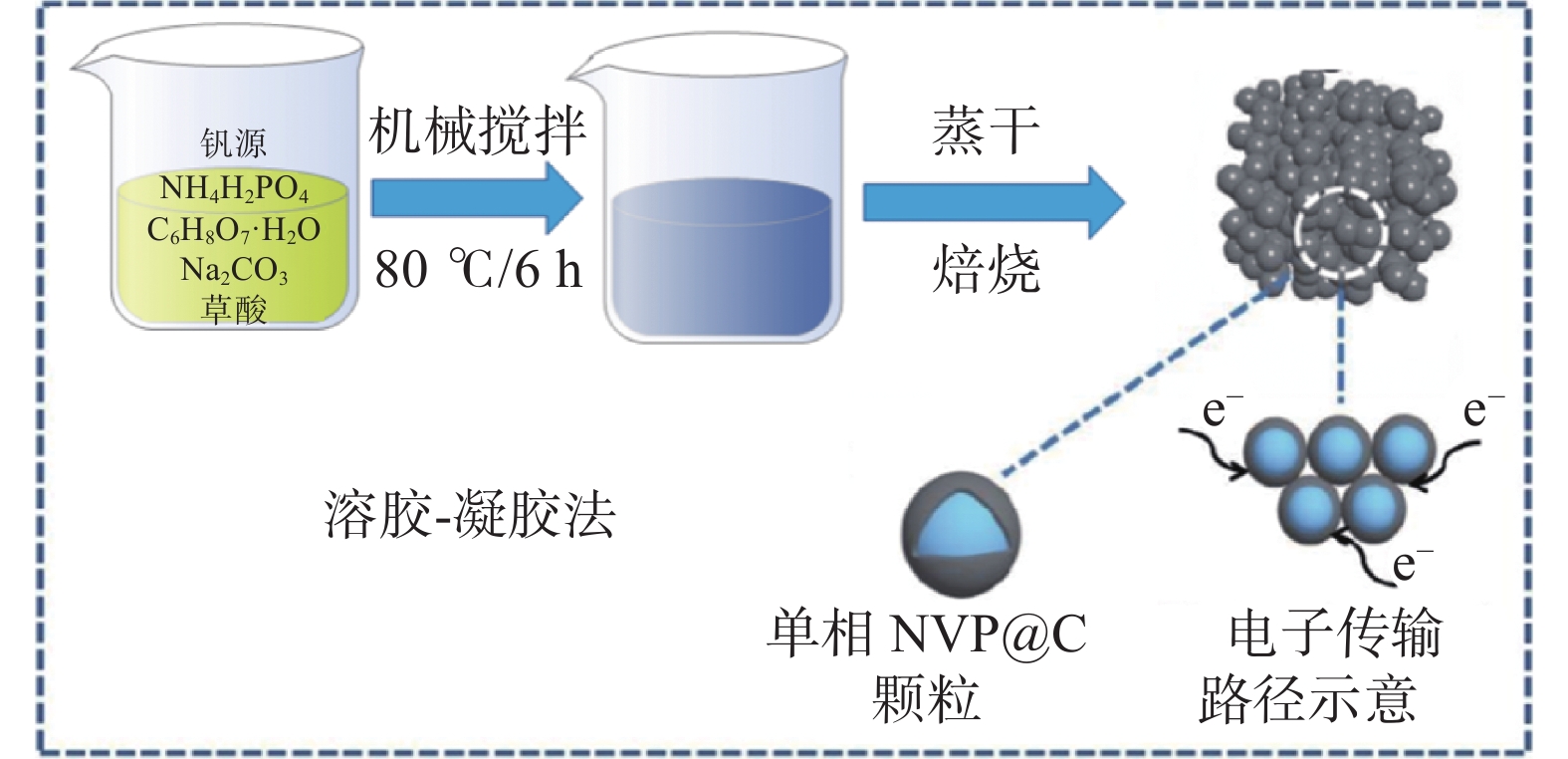
摘要: 以不同提钒工艺制备的中间产物多钒酸铵NH4V3O8(APV)和高纯五氧化二钒为钒源,Na2CO3、NH4H2PO4、柠檬酸分别作钠源、磷源、碳源,采用溶胶凝胶法合成了一系列Na3V2(PO4)3/C正极材料。通过XRD、SEM、电池测试系统和电化学工作站等详细研究了不同钒源对Na3V2(PO4)3/C正极材料的影响。结果显示:钠法提钒多钒酸铵为钒源制备的NaH-NVP正极材料呈现较好的循环性能和优异的高倍率性能,在5C和10C高倍率下分别表现出98 mAh/g和64 mAh/g的可逆容量。此项研究拓展了合成磷酸钒钠材料的钒源选择,对降低磷酸钒钠的制备成本具有积极意义。Abstract: A series of Na3V2(PO4)3/C anode materials were synthesized by sol-gel method, using the intermediate products ammonium polyvanadate (APV, NH4V3O8) prepared by different vanadium extraction processes and high-purity vanadium pentoxide as vanadium sources, and Na2CO3, NH4H2PO4, and citric acid as sodium, phosphorus, and carbon sources, respectively. The effects of different vanadium sources on Na3V2(PO4)3/C anode materials were investigated in detail through XRD, SEM, battery testing system and electrochemical workstation. The results show that the Na3V2(PO4)3/C (NaH-NVP) cathode materials prepared by ammonium polyvanadate from sodium method for vanadium extraction as the vanadium source present superior high-rate performance, i.e., reversible capacities of 98 mAh/g and 64 mAh/g at 5C and 10C, respectively. The research has expanded the selection of vanadium sources for synthesizing sodium vanadium phosphate materials, which has a positive significance in reducing the preparation cost of sodium vanadium phosphate.
-
Key words:
- vanadium source /
- sol-gel method /
- sodium vanadium phosphate /
- cathode materials
-
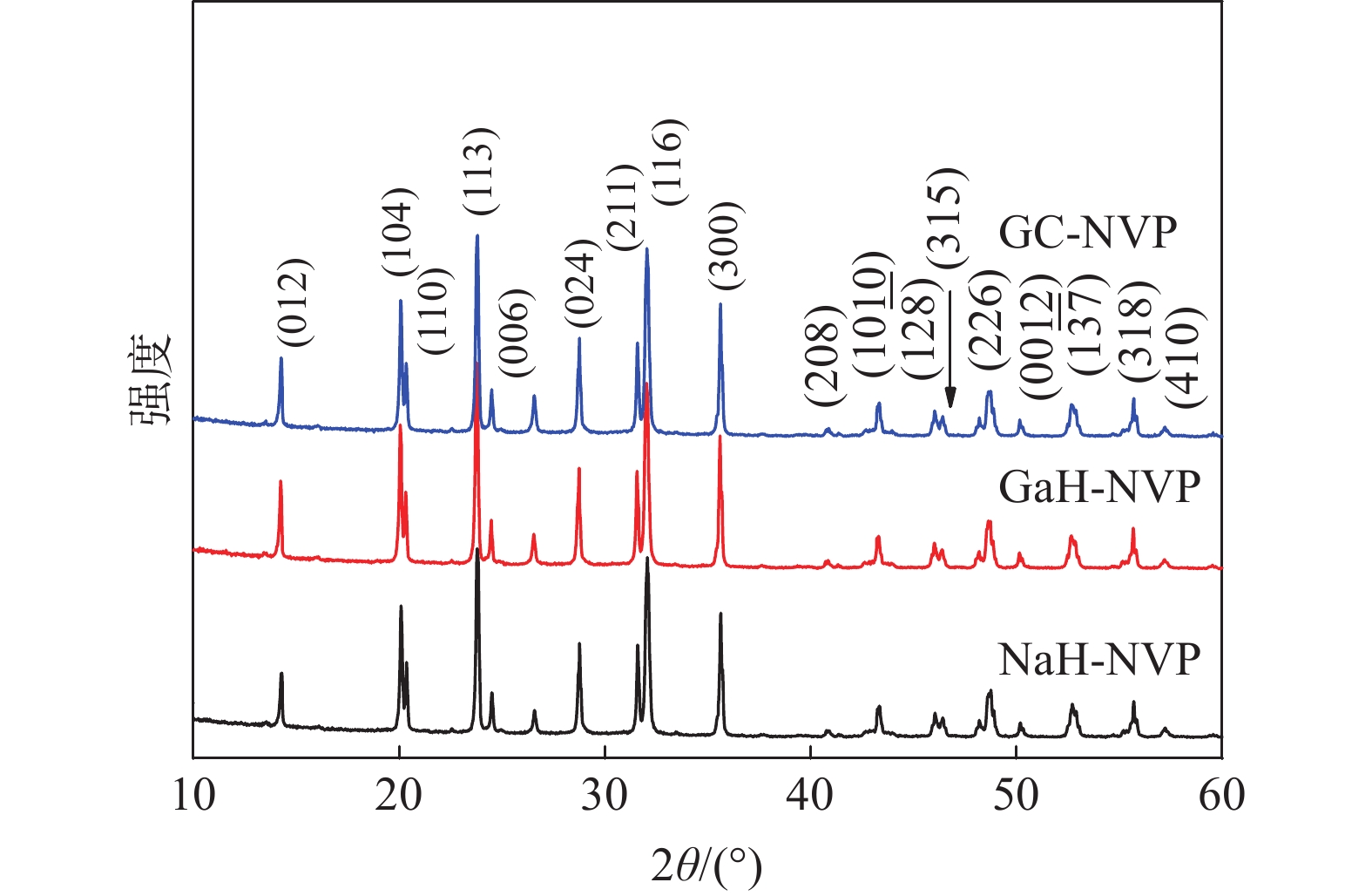
表 1 不同工艺钒源的成分表
Table 1. Compositions table of V2O5 from different processes
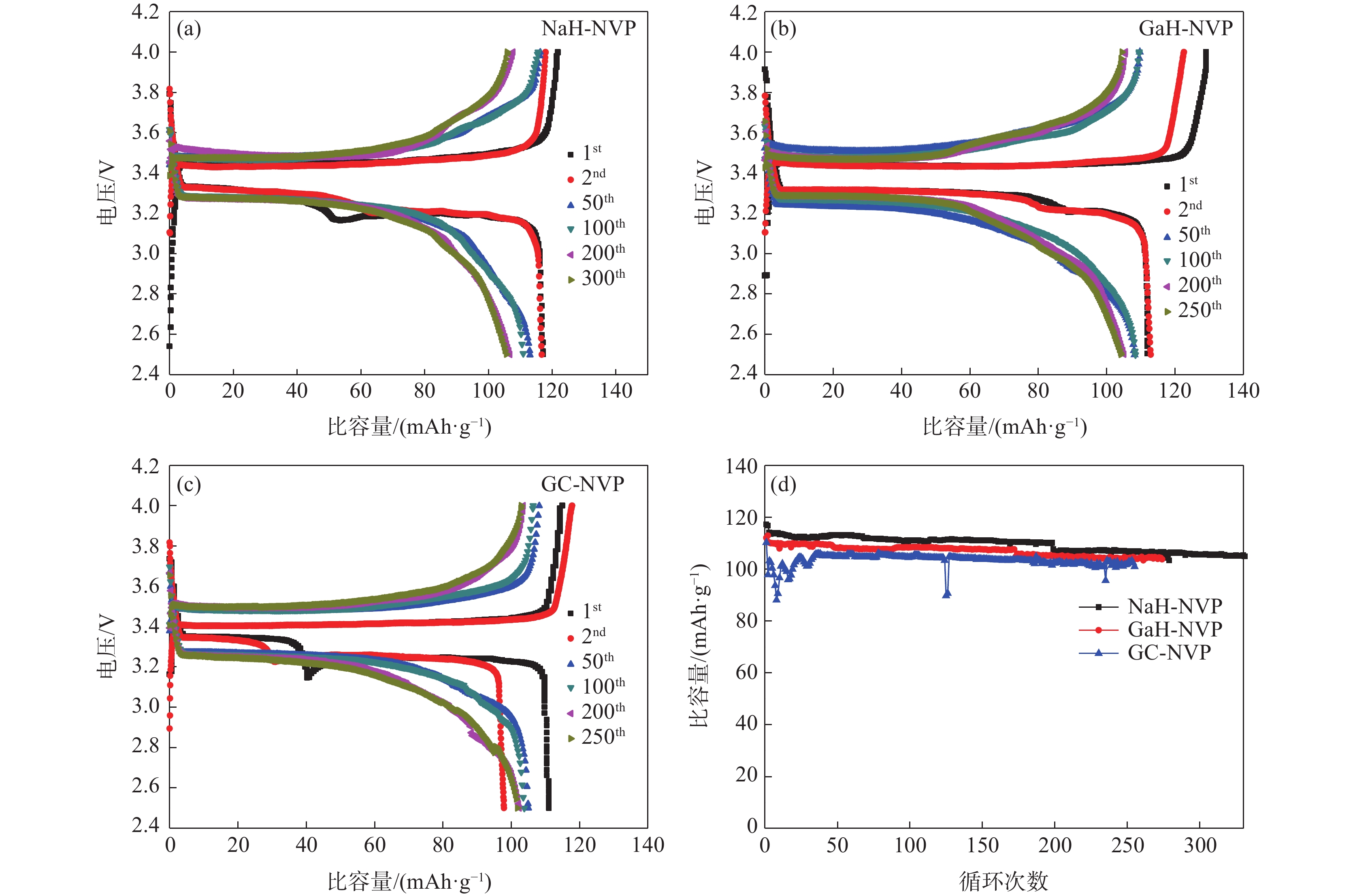
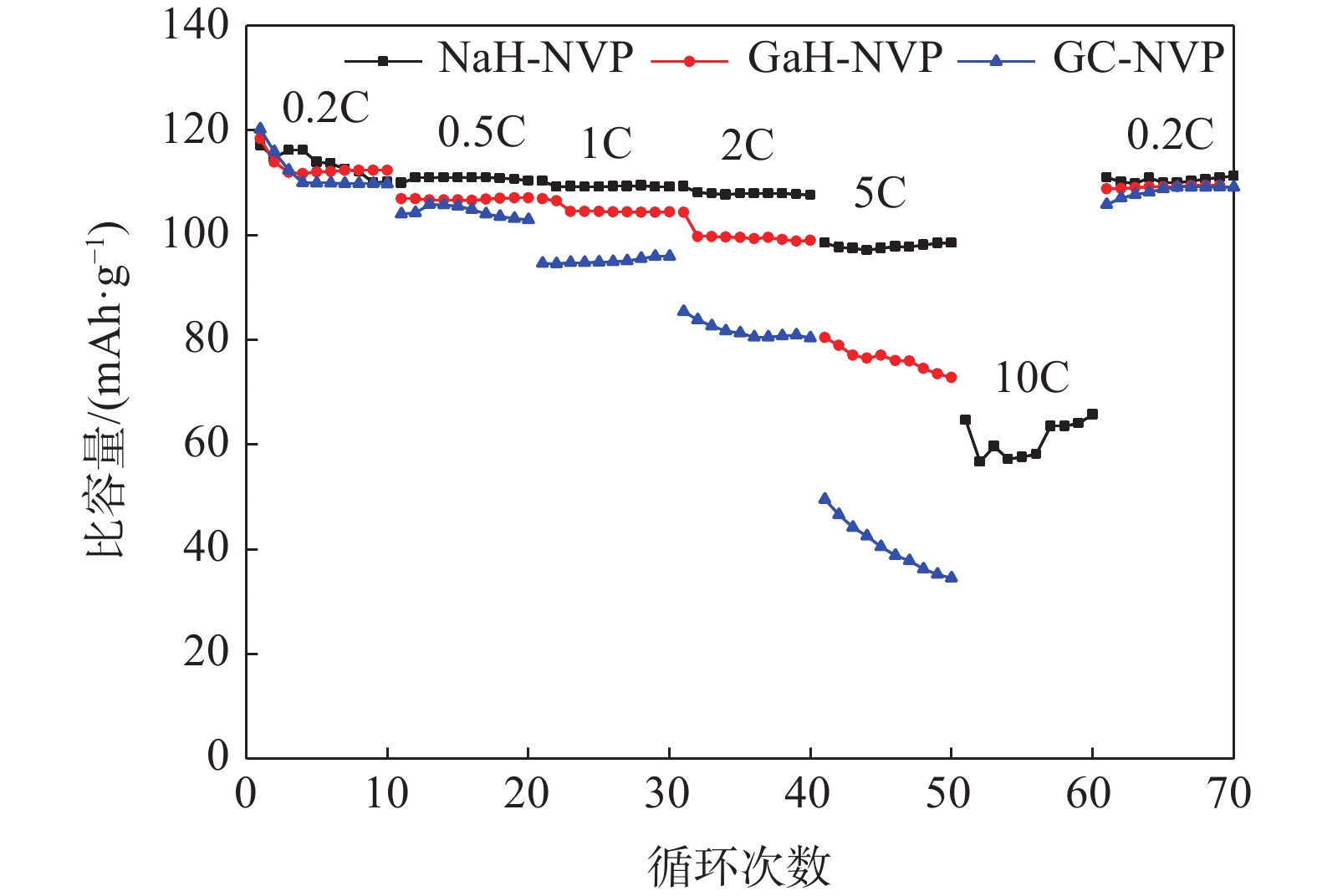
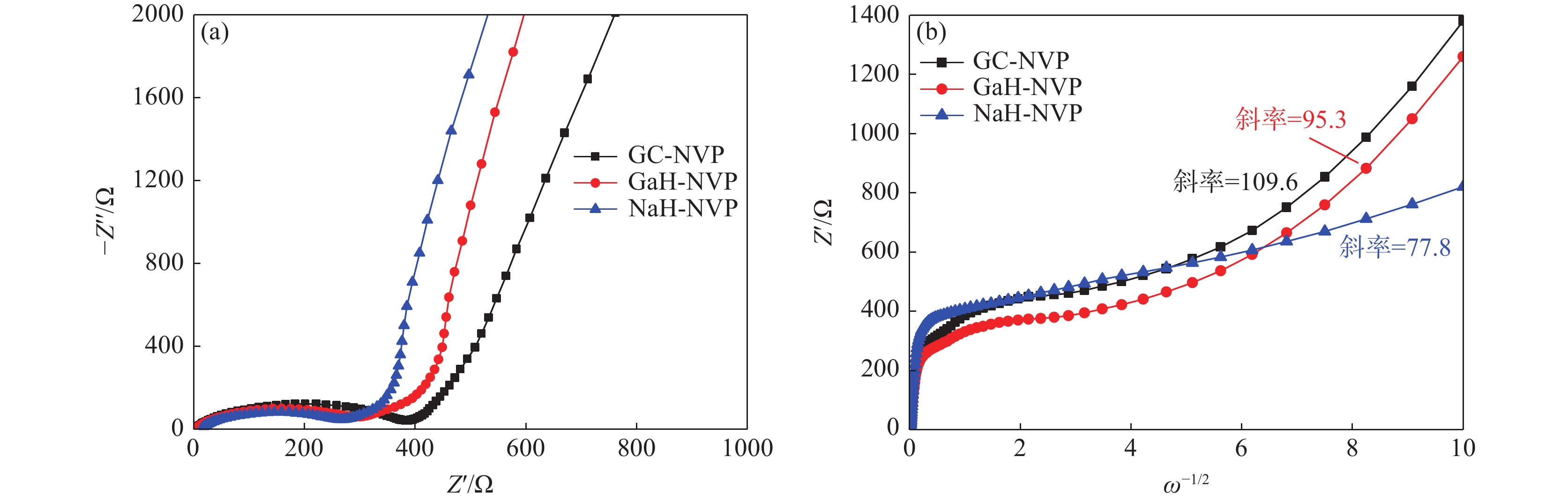
% V Ca Na Si Mn K Fe Cr 钙法APV 49.73 0.02 0.012 0.013 0.081 0.122 0.046 <0.01 钠法APV 50.16 0.248 0.082 <0.01 0.072 0.013 0.065 高纯V2O5 55.95 0.010 0.013 <0.01 <0.01 0.010 表 2 NaH-NVP、GaH-NVP和GC-NVP材料的循环性能和倍率性能
Table 2. Cycling and rate performances of NaH-NVP, GaH-NVP and GC-NVP materials
循环性能/(mAh·g−1) 倍率性能/(mAh·g−1) 1st 150th 0.2C 0.5C 1C 2C 5C 10C 回到0.2C NaH-NVP 117 111 117 110 110 109 98 64 111 GaH-NVP 112 107 118 107 106 104 80 108 GC-NVP 111 104 120 104 95 85 49 106 -
[1] XIE H J, ZHENG D F, LUO X, et al. Research progress on key materials for sodium ion batteries[J]. Zhejiang Chemical Industry, 2023,54(12):8-14. (谢浩杰, 郑冬芳, 罗霞, 等. 钠离子电池关键材料研究进展[J]. 浙江化工, 2023,54(12):8-14. doi: 10.3969/j.issn.1006-4184.2023.12.002XIE H J, ZHENG D F, LUO X, et al. Research progress on key materials for sodium ion batteries[J]. Zhejiang Chemical Industry, 2023, 54(12): 8-14. doi: 10.3969/j.issn.1006-4184.2023.12.002 [2] XIANG X, ZHANG K, CHEN J. Recent advances and prospects of cathode materials for sodium-ion batteries[J]. Advanced Materials, 2015,27(36):5343-5364. doi: 10.1002/adma.201501527 [3] ROJO T, HU Y S, FORSYTH M, et al. Sodium-ion batteries[J]. Advanced Energy Materials, 2018,8(17):1800880. doi: 10.1002/aenm.201800880 [4] HWANG J Y, MYUNG S T, SUN Y K. Sodium-ion batteries: present and future[J]. Chemical Society Reviews, 2017,46(12):3529-3614. doi: 10.1039/C6CS00776G [5] CHEN X, WANG Y, WIADEREK K, et al. Super charge separation and high voltage phase in NaxMnO2[J]. Advanced Functional Materials, 2018,28(50):1805105.1-1805105.12. [6] YOSHIDA H, YABUUCHI N, KOMABA S. NaFe0.5Co0.5O2 as high energy and power positive electrode for Na-ion batteries[J]. Electrochemistry Communications, 2013,34:60-63. doi: 10.1016/j.elecom.2013.05.012 [7] MOLENDA J, BASTER D, MOLENDA M, et al. Anomaly in the electronic structure of the NaxCoO2-y cathode as a source of its step-like discharge curve[J]. Physical Chemistry Chemical Physics, 2014,16(28):14845-14857. doi: 10.1039/c3cp55223c [8] MOLENDA J, BASTER D, GUTOWSKA M U, et al. Electronic origin of the step-like character of the discharge curve for NaxCoO2-y cathode[J]. Functional Materials Letters, 2014,7(6):1440009. doi: 10.1142/S1793604714400098 [9] MAO J, LIU X, LIU J, et al. P2-type Na2/3Ni1/3Mn2/3O2 cathode material with excellent rate and cycling performance for sodium-ion batteries[J]. Journal of The Electrochemical Society, 2019,166(16):A3980-A3986. doi: 10.1149/2.0211916jes [10] WU X, WU C, WEI C, et al. Highly crystallized Na2CoFe(CN)6 with suppressed lattice defects as superior cathode material for sodium-ion batteries[J]. Acs Applied Materials & Interfaces, 2016,8(8):5393. [11] MUKHERJEE A, SHARABANI T, PERELSHTEIN I, et al. Three-sodium ion activity of hollow spherical Na3V2(PO4)2F3 cathode: new aspects on high capacity and stability[J]. Batteries & Supercaps, 2019,3(1):52-55. [12] WANG D, WU Y, LÜ J, et al. Carbon encapsulated maricite NaFePO4 nanoparticles as cathode material for sodium-ion batteries[J]. Colloids and Surfaces A Physicochemical and Engineering Aspects, 2019,583:123957. doi: 10.1016/j.colsurfa.2019.123957 [13] ZHU C, SONG K, AKEN P A V, et al. Carbon-coated Na3V2(PO4)3 embedded in porous carbon matrix: an ultrafast Na-storage cathode with the potential of outperforming Li cathodes[J]. Nano Letters, 2014,14(4):2175-2180. doi: 10.1021/nl500548a [14] WANG Y, ZHANG X, HE W, et al. A review for the synthesis methods of lithium vanadium phosphate cathode materials[J]. Journal of Materials Science: Materials in Electronics, 2017,28(24):18269-18295. doi: 10.1007/s10854-017-7834-1 [15] WANG K, YAO Z C, MA B Z, et al. Current status and research progress of vanadium deposition process[J]. Hebei Metallurgy, 2023(10):1-7. (王宽, 姚志超, 马保中, 等. 沉钒工艺现状和研究进展[J]. 河北冶金, 2023(10):1-7.WANG K, YAO Z C, MA B Z, et al. Current status and research progress of vanadium deposition process[J]. Hebei Metallurgy, 2023(10): 1-7. [16] LIM S J, HAN D W, NAM D H, et al. Structural enhancement of Na3V2(PO4)3/C composite cathode materials by pillar ion doping for high power and long cycle life sodium-ion batteries[J]. Journal of Materials Chemistry A, 2014,2(46):19623-19632. doi: 10.1039/C4TA03948C [17] WANG M Y, GUO J Z, WANG Z W, et al. Isostructural and multivalent anion substitution toward improved phosphate cathode materials for sodium-ion batteries[J]. Small, 2020,16(16):e1907645. doi: 10.1002/smll.201907645 [18] SHEN W, LI H, GUO Z, et al. Improvement on high-rate performance of Mn-doped Na3V2(PO4)3/C as cathode materials for sodium ion batteries[J]. RSC Advances, 2016,6(75):71581-71588. doi: 10.1039/C6RA16515J [19] ZHU Y L. Preparation and modification of sodium vanadium phosphate positive electrode material for sodium ion batteries[D]. Harbin: Harbin Institute of Technology, 2020. (朱昱龙. 钠离子电池磷酸钛钒钠正极材料的制备及改性研究[D]. 哈尔滨: 哈尔滨工业大学, 2020.ZHU Y L. Preparation and modification of sodium vanadium phosphate positive electrode material for sodium ion batteries[D]. Harbin: Harbin Institute of Technology, 2020. [20] ZHANG B W. Preparation and sodium storage properties of Na3V2(PO4)3 as positive electrode material for sodium ion batteries[D]. Tangshan: North China University of Science and Technology, 2021. (张博文. 钠离子电池正极材料Na3V2(PO4)3的制备及储钠性能研究[D]. 唐山: 华北理工大学, 2021.ZHANG B W. Preparation and sodium storage properties of Na3V2(PO4)3 as positive electrode material for sodium ion batteries[D]. Tangshan: North China University of Science and Technology, 2021. [21] YAN W X, WANG X J, HAN Y, et al. Green large-scale preparation of Na3V2(PO4)3 with good rate capability and long cycling lifespan for sodium-ion batteries[J]. ACS Sustainable Chem. Eng, 2024,12(6):2394-2403. doi: 10.1021/acssuschemeng.3c07336 -





 下载:
下载: