Preparation of VAl55 alloy by vacuum aluminothermy with V 2O3 powder
-
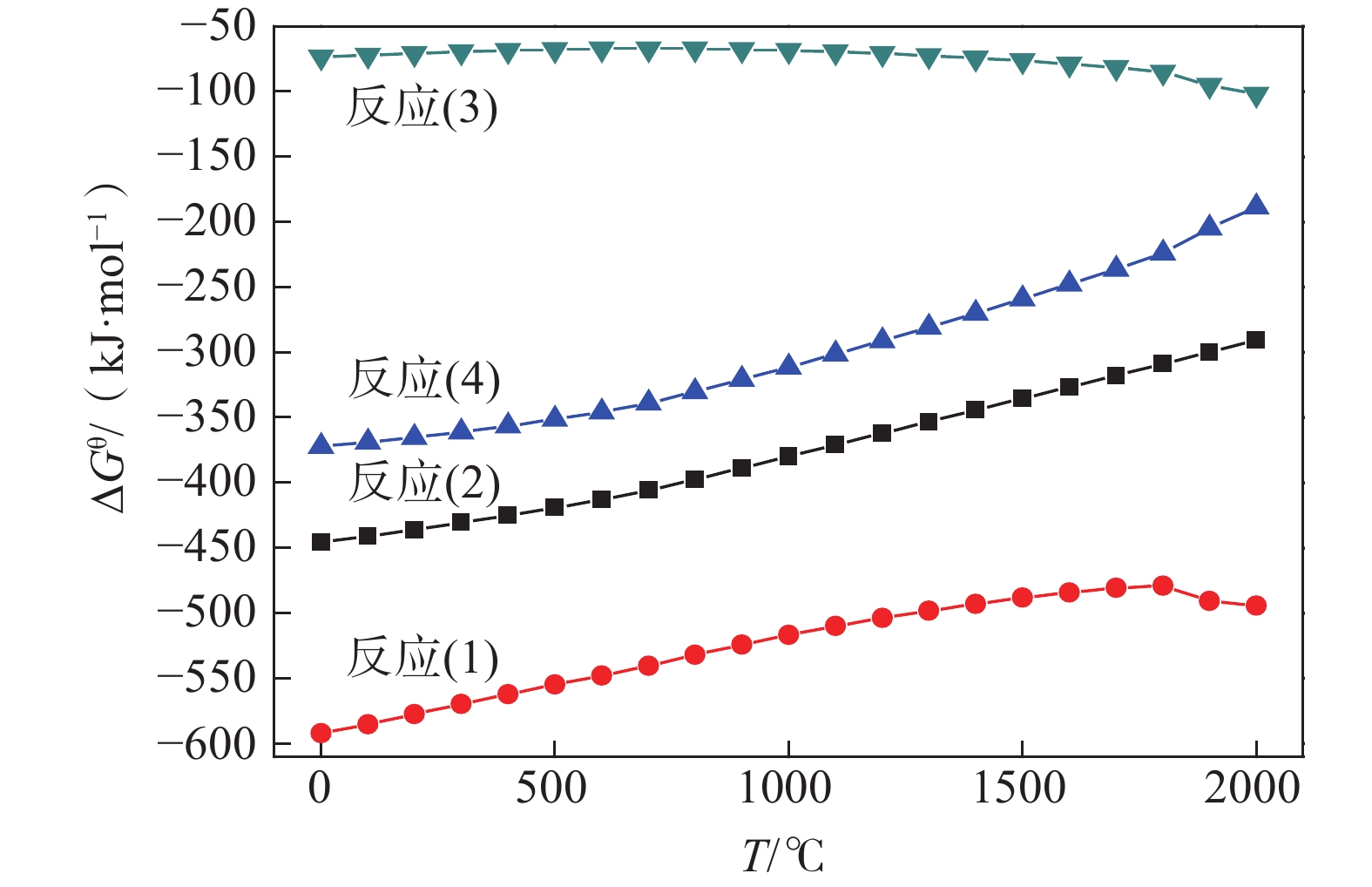
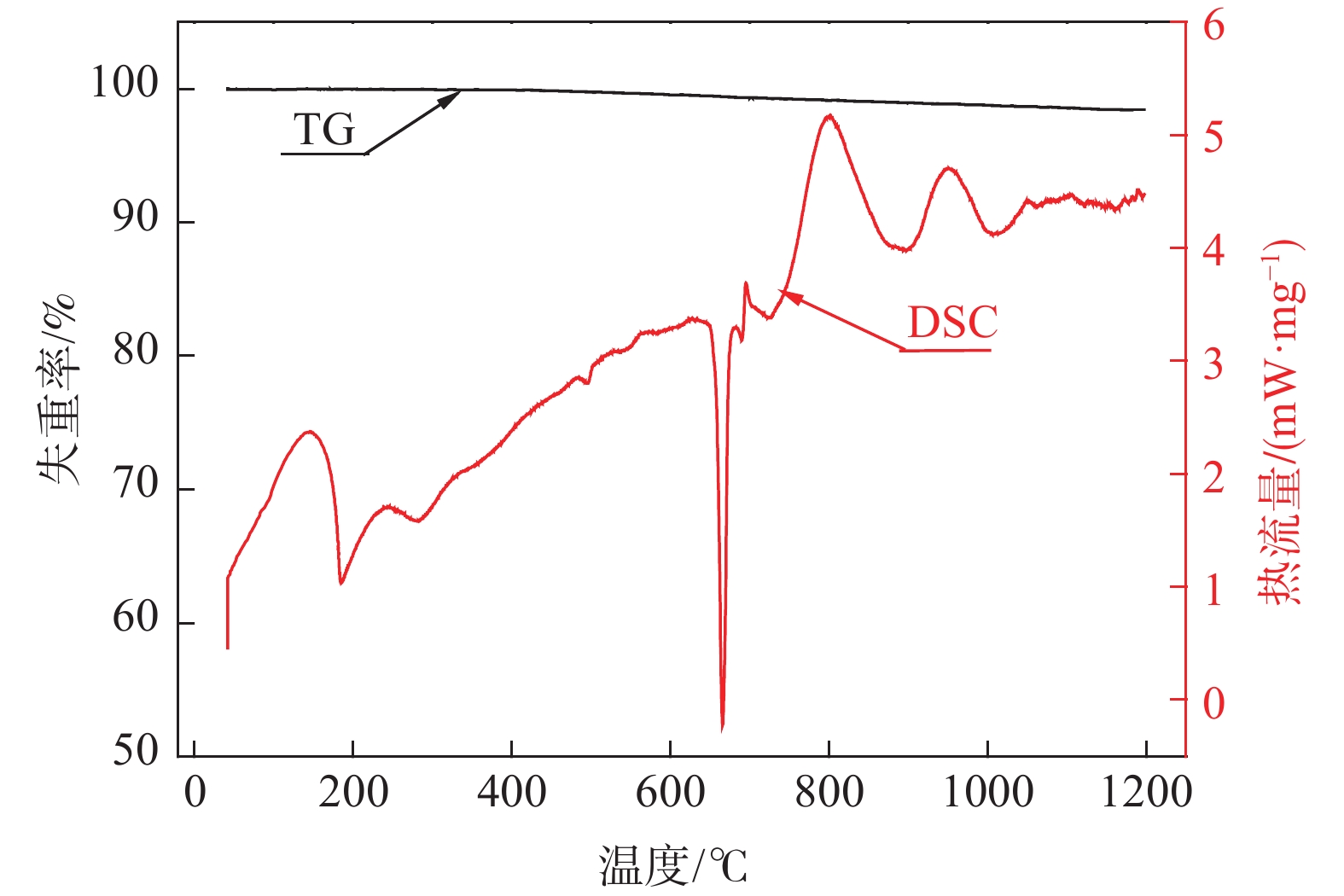
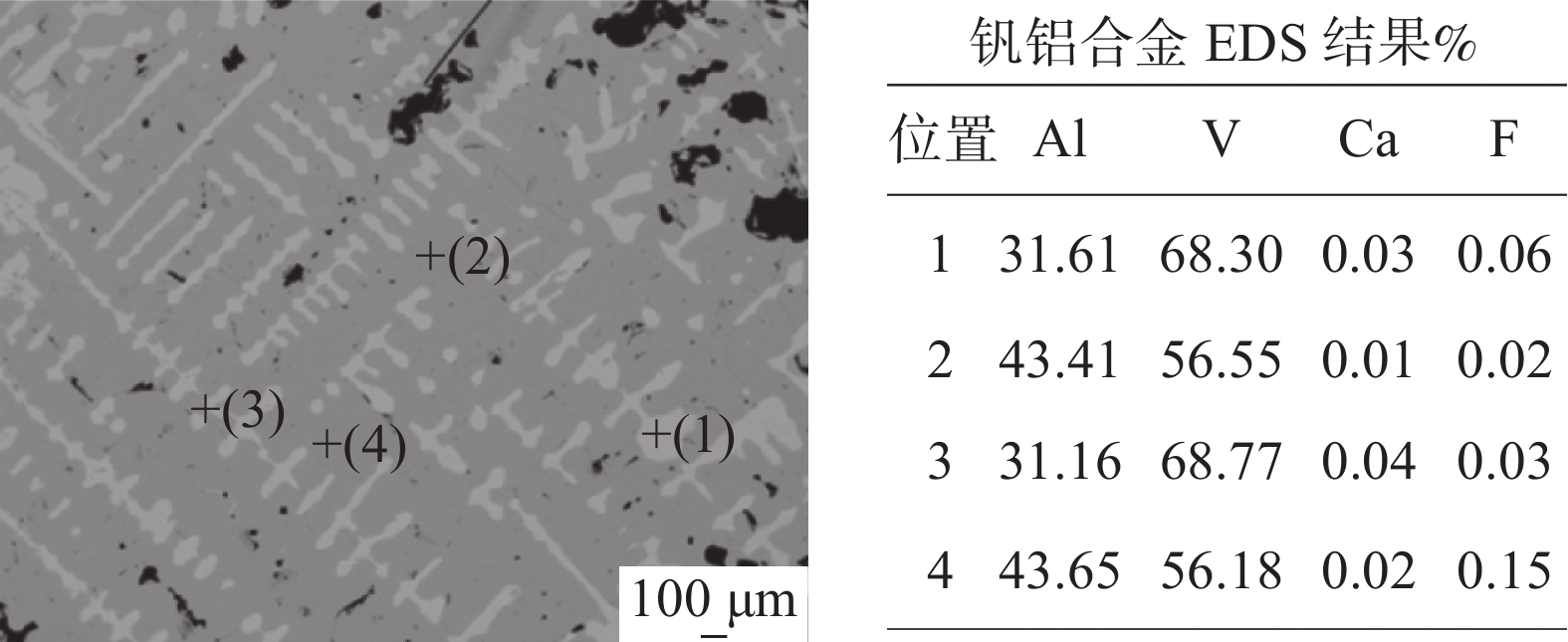
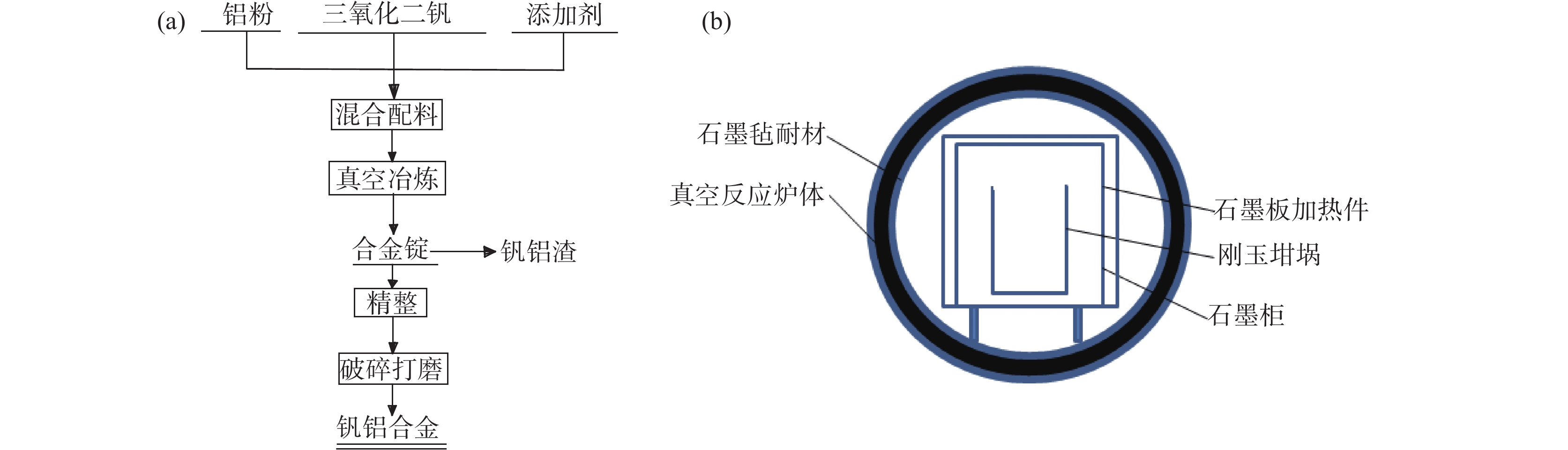
摘要: 以V2O3和Al粉为原料,真空铝热还原法制备AlV55合金,利用热力学软件和差热分析法对铝热法制备钒铝合金的过程进行了分析,结果表明:热力学计算常温下铝热还原反应可将V2O3一步还原为V;实际还原过程为分步进行,开始温度为749 ℃,且属于液-固反应范畴;单位热效应为
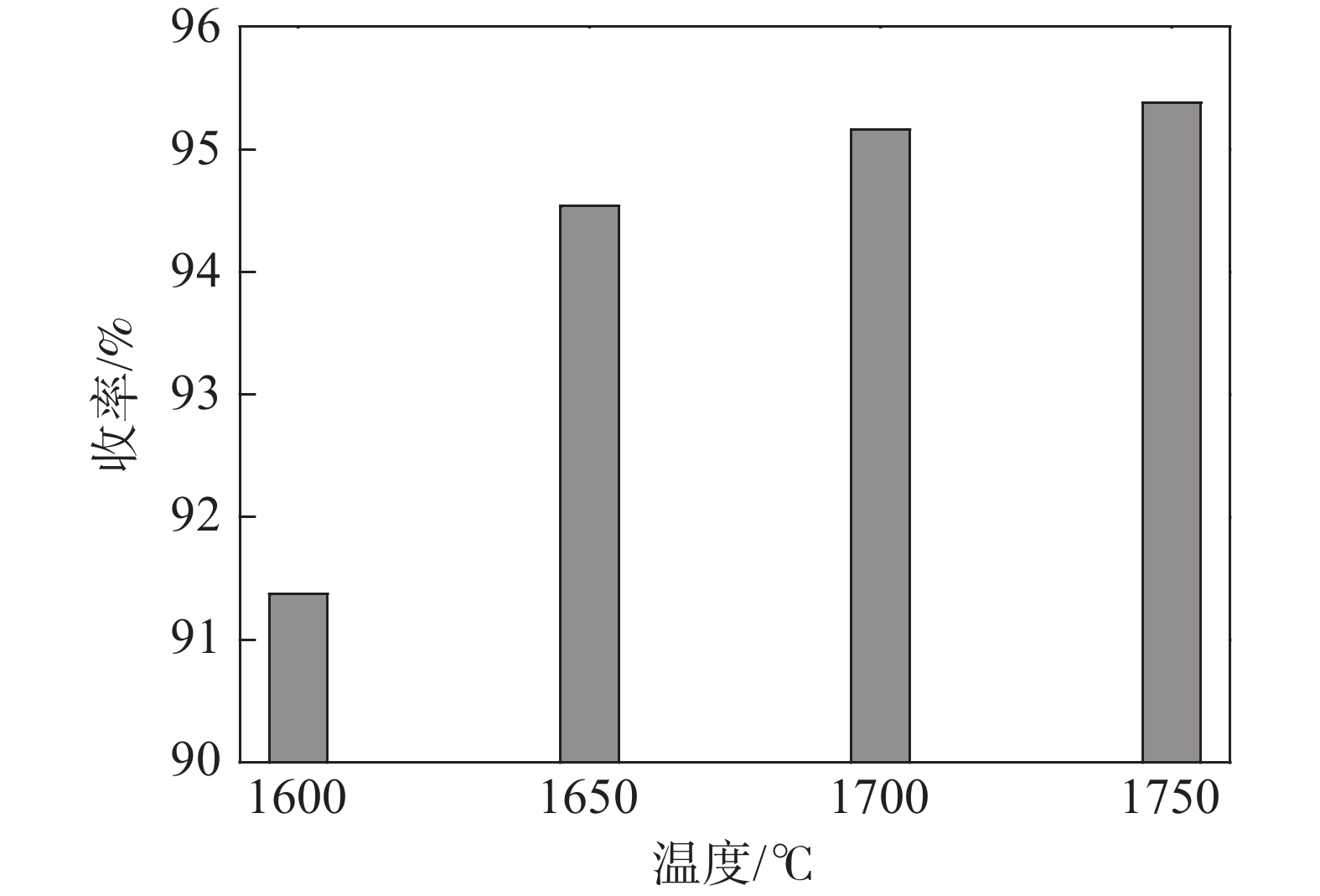
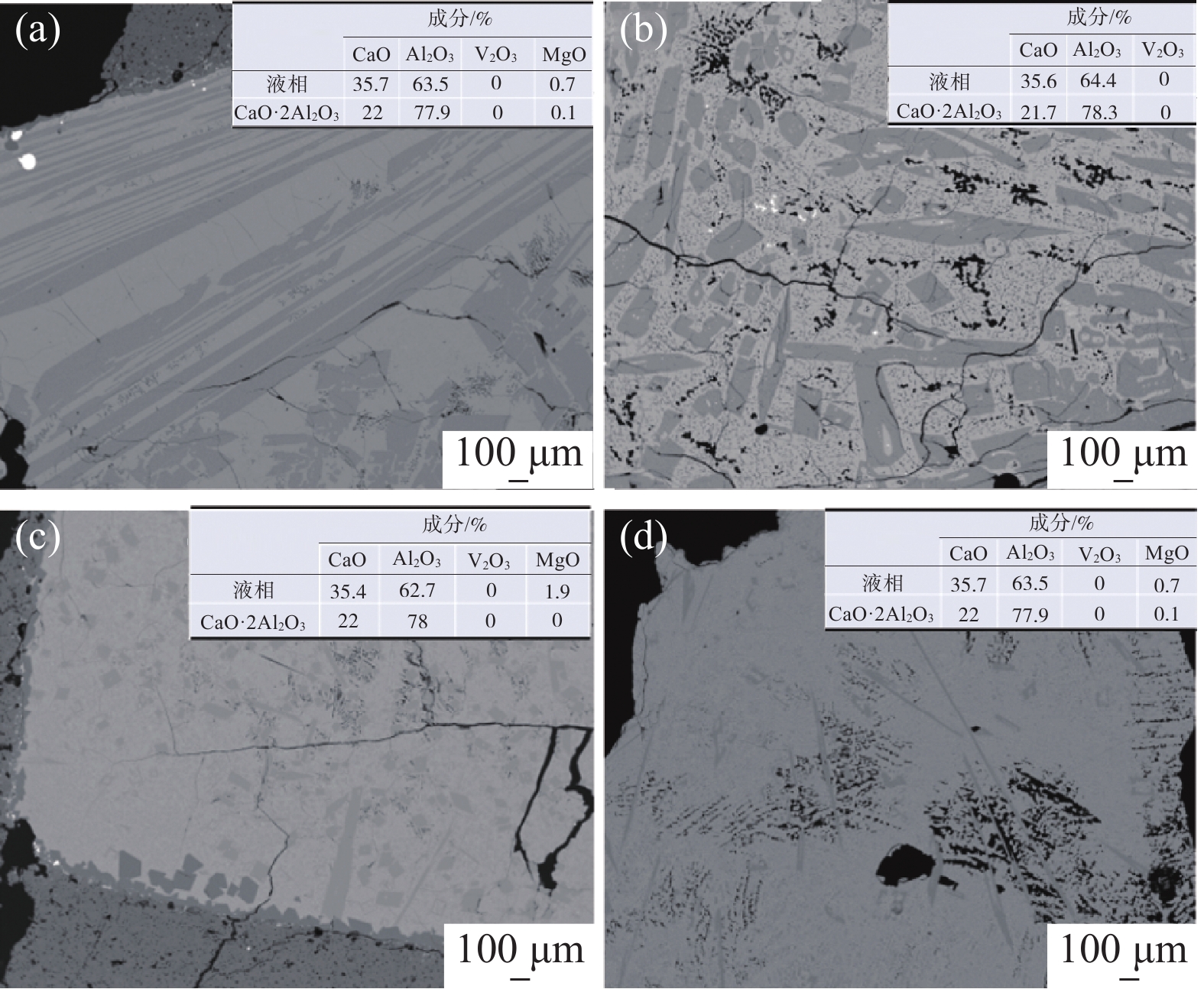
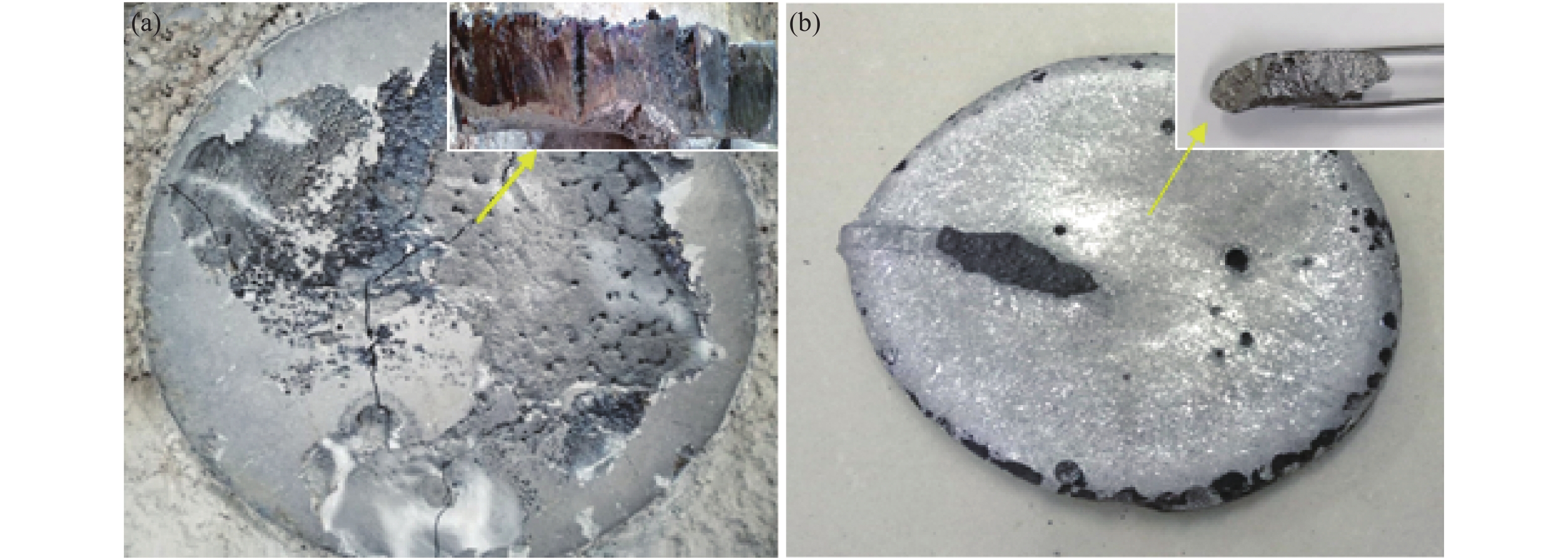
2239.65 J/g,绝热燃烧温度为1728.7 ℃。研究了真空加热过程中保温温度和造渣剂氧化钙加入量对钒收率的影响,当保温温度超过1650 ℃时,钒铝合金的钒收率可达到95%左右,钒铝合金锭无裂隙和氧化膜,钒铝合金的成品率显著提升。此外,还确定了CaO/Al2O3比值对炉渣粘度的影响,当造渣剂用量超过22.5%时,渣金的分离效果显著提升。Abstract: Using V2O3 and Al powder as raw materials, AlV55 alloy was prepared by vacuum aluminothermic reduction method. The process of preparing vanadium-aluminum alloy by aluminothermic method was analyzed using thermodynamic software and differential thermal analysis method. The results showed that the thermodynamic calculation of aluminothermic reduction reaction at room temperature can reduce V2O3 to V in one step. The actual reduction process is carried out in stages, with a starting temperature of 749 ℃, belonging to the category of liquid-solid reactions. The unit thermal effect is2239.65 J/g, and the adiabatic combustion temperature is1728.7 ℃. The effects of insulation temperature and the amount of slag forming agent calcium oxide added during vacuum heating on vanadium yield were studied. When the insulation temperature exceeded1650 ℃, the vanadium yield of vanadium-aluminum alloy could reach about 95%. The vanadium-aluminum alloy ingot had no cracks or oxide film, and the yield of vanadium-aluminum alloy was significantly improved. In addition, the influence of CaO/Al2O3 ratio on slag viscosity was also determined. When the amount of slag forming agent exceeded 22.5%, the separation effect of slag and metal was significantly improved.-
Key words:
- vanadium-aluminum alloy /
- thermite reduction /
- slag-forming agent
-
表 1 试验原料的主要化学成分
Table 1. Main chemical components of experimental raw materials
% 原料 Fe Si Al C TV Ca V2O3 0.127 0.016 0.060 0.015 65.3 铝粉 0.07 0.05 99.75 氧化钙 0.075 0.132 0.050 0.008 71.51 表 2 热力学参数的计算结果
Table 2. Calculation results of thermodynamic parameters
△G$ _{298}^{{\theta}} $/(kJ·mol−1) q/(J·g−1) Tad/ ℃ −435.75 2239.65 1728.70 -
[1] CHEN J, YAN F Y, CHEN B B, et al. Assessing the tribocorrosion performance of Ti-6Al-4V, 316 stainless steel and Monel K500 alloys in artificial seawater[J]. Materials and Corrosion, 2013,64(5):394-401. doi: 10.1002/maco.201106249 [2] KOSTOV A, ZIVKOVIC D, FRIEDRICH B. Thermodynamic study of Ti-V and Al-V systems using FactSage[J]. Journal of Mining and Metallurgy B: Metallurgy, 2006,42(1):57-65. doi: 10.2298/JMMB0601057K [3] SHI W J, WANG S H, LI W L, et al. The phase transition of calcium phosphate coatings deposited on a Ti-6Al-4V substrate by an electrolytic method[J]. Journal of alloys and compounds, 2007,434:693-696. [4] LI Y, CHEN C, HAN T, et al. Microstructures and oxidation behavior of NiCrAlCoY-Al composite coatings on Ti-6Al-4V alloy substrate via high-energy mechanical alloying method[J]. Journal of Alloys and Compounds, 2017,697:268-281. doi: 10.1016/j.jallcom.2016.10.171 [5] LIU Y, WANG D, DENG C, et al. Novel method to fabricate Ti-Al intermetallic compound coatings on Ti-6Al-4V alloy by combined ultrasonic impact treatment and electrospark deposition[J]. Journal of Alloys and Compounds, 2015,628:208-212. doi: 10.1016/j.jallcom.2014.12.144 [6] KURTZ R J, ABE K, CHERNOV V M, et al. Recent progress on development of vanadium alloys for fusion[J]. Journal of nuclear materials, 2004,329:47-55. [7] CHENG C, DOU Z H, ZHANG T A, et al. Synthesis of as-cast Ti-A-V alloy from titanium-rich material by thermite reduction[J]. JOM, 2017,69(10):1818-1823. doi: 10.1007/s11837-017-2467-7 [8] MA H S. Vacuum smelting of titanium and refractory metals[M]. Changsha: Central South University Press, 2010. (马宏声. 钛及难熔金属真空熔炼[M]. 长沙: 中南大学出版社, 2010.MA H S. Vacuum smelting of titanium and refractory metals[M]. Changsha: Central South University Press, 2010. [9] LI Y J, WAMG J, WU H Q. XRD and TEM analysis of Fe3Al alloy layer on the surface of the calorized steel[J]. Materials research bulletin, 2001,36(13-14):2389-2394. doi: 10.1016/S0025-5408(01)00721-8 [10] LI S Y. Application and prospect of vanadium[J]. Rare Metals and Cemented Carbides, 2000,141:58-61. (刘世友. 钒的应用与展望[J]. 稀有金属与硬质合金, 2000,141:58-61. doi: 10.3969/j.issn.1004-0536.2000.02.016LI S Y. Application and prospect of vanadium[J]. Rare Metals and Cemented Carbides, 2000, 141: 58-61. doi: 10.3969/j.issn.1004-0536.2000.02.016 [11] ZHANG E, WANG X, HAN Y. Research status of biomedical porous Ti and its alloy in China[J]. Acta Metall Sin, 2017,53(12):1555-1567. [12] RAHMATI B, SARHAN A D, BASIRUN W J, et al. Ceramic tantalum oxide thin film coating to enhance the corrosion and wear characteristics of Ti6Al4V alloy[J]. Journal of Alloys and Compounds, 2016,676:369-376. doi: 10.1016/j.jallcom.2016.03.188 [13] LA P Q, LU X F, SHEN D, et al. Study on high grade V-Al alloy prepared by aluminothermic reaction[J]. Powder Metallurgy Technology, 2012,30(5):371-375. (喇培清, 卢学峰, 申达, 等. 铝热法制备高钒铝合金的研究[J]. 粉末冶金技术, 2012,30(5):371-375. doi: 10.3969/j.issn.1001-3784.2012.05.010LA P Q, LU X F, SHEN D, et al. Study on high grade V-Al alloy prepared by aluminothermic reaction[J]. Powder Metallurgy Technology, 2012, 30(5): 371-375. doi: 10.3969/j.issn.1001-3784.2012.05.010 [14] DUAN S C, WANG Z Q, GUO H J, et al. Study on thermodynamic and kinetics for preparation of vanadium-aluminum alloy based on thermite reaction[J]. Powder Metallurgy Technology, 2017, 38(6): 47-54. ) (段生朝, 王竹青, 郭汉杰, 等. 铝热法制备钒铝合金热力学及动力学研究[J]. 钢铁钒钛, 2017, 38(6): 47-54.DUAN S C, WANG Z Q, GUO H J, et al. Study on thermodynamic and kinetics for preparation of vanadium-aluminum alloy based on thermite reaction[J]. Powder Metallurgy Technology, 2017, 38(6): 47-54. ) [15] HUANG D X. Extraction of vanadium to make steel[M]. Beijing: Metallurgical Industry Press, 2000. (黄道鑫. 提钒炼钢[M]. 北京: 冶金工业出版社, 2000 .HUANG D X. Extraction of vanadium to make steel[M]. Beijing: Metallurgical Industry Press, 2000. [16] YIN D F, SUN Z H, CHEN H J, et al. Preparation of AlV55 alloy by aluminothermic reduction[J]. Iron Steel Vanadiom Titanium, 2017,38(9):42-45. (尹丹凤, 孙朝晖, 陈海军, 等. 铝热还原法制备AlV55合金的研究[J]. 钢铁钒钛, 2017,38(9):42-45. doi: 10.7513/j.issn.1004-7638.2017.05.008YIN D F, SUN Z H, CHEN H J, et al. Preparation of AlV55 alloy by aluminothermic reduction[J]. Iron Steel Vanadiom Titanium, 2017, 38(9): 42-45. doi: 10.7513/j.issn.1004-7638.2017.05.008 [17] LA P Q, LU X F, SHEN D, et al. Study on high grade V-Al alloy prepared by aluminothermic reaction[J]. Powder Metallurgy Technology, 2012,30(5):371-375. (喇培清, 卢学峰, 申达, 等. 铝热法制备高钒铝合金的研究[J]. 粉末冶金技术, 2012,30(5):371-375. doi: 10.3969/j.issn.1001-3784.2012.05.010LA P Q, LU X F, SHEN D, et al. Study on high grade V-Al alloy prepared by aluminothermic reaction[J]. Powder Metallurgy Technology, 2012, 30(5): 371-375. doi: 10.3969/j.issn.1001-3784.2012.05.010 [18] WANG Y G, WEN B C, SHA Z Z. Compararive analysis on properties of V-Al alloy[J]. Metallic Functional Materials, 2020,27(6):51-56. (王永钢, 文本超, 沙志忠. 钒铝合金物性浅析[J]. 金属功能材料, 2020,27(6):51-56.WANG Y G, WEN B C, SHA Z Z. Compararive analysis on properties of V-Al alloy[J]. Metallic Functional Materials, 2020, 27(6): 51-56. [19] CHEN X M. Physical chemistry of pyrometallurgical process[J]. Beijing: Metallurgical Industry Press, 1994. (陈新民. 火法冶金过程物理化学[M]. 北京: 冶金工业出版社, 1994.CHEN X M. Physical chemistry of pyrometallurgical process[J]. Beijing: Metallurgical Industry Press, 1994. [20] CHEN H J, SHI Q H, WANG Y G, et al. Technical discussion on improving AlV55 yield[J]. Iron Steel Vanadiom Titanium, 2019,40(6):18-23. (陈海军, 师启华, 王永钢, 等. 提高AlV55成品率技术探讨[J]. 钢铁钒钛, 2019,40(6):18-23. doi: 10.7513/j.issn.1004-7638.2019.06.004CHEN H J, SHI Q H, WANG Y G, et al. Technical discussion on improving AlV55 yield[J]. Iron Steel Vanadiom Titanium, 2019, 40(6): 18-23. doi: 10.7513/j.issn.1004-7638.2019.06.004 [21] ZHANG S X, LI L J, LI J J, et al. Study on the production technology of vanadium aluminum alloy by electro-aluminothermic process[J]. Hebei Metalluegy, 2018(9):23-25. (张苏新, 李兰杰, 李九江等. 钒铝合金电铝热法生产技术研究[J]. 河北冶金, 2018(9):23-25.ZHANG S X, LI L J, LI J J, et al. Study on the production technology of vanadium aluminum alloy by electro-aluminothermic process[J]. Hebei Metalluegy, 2018(9): 23-25. -





 下载:
下载: