Study on the diffusion behavior of carbon during solid-state decarbonization process
-
摘要: 为研究Fe-C-Mn合金薄带固态脱碳过程脱碳效果及Mn含量对C原子扩散作用,以1 mm厚的Fe-2.7%C-(5%,12%)Mn合金成分薄带为研究对象进行固态脱碳试验,利用lammps软件开展分子动力学模拟试验,探索Mn含量对C原子扩散的定性规律。结果表明:分子动力学模拟结果同固态脱碳试验结果计算扩散激活能基本一致,5%Mn在1223、1323 K和1363 K温度下脱碳试验结果和分子动力学模拟理论结果得到的C原子的扩散激活能分别为78.549 kJ·mol−1和83.805 kJ·mol−1。在1363 K进行固态脱碳试验,5%Mn和12%Mn合金薄带5~20 min主要限制性环节为内部碳扩散,12%Mn脱碳效果不如5%Mn,表明Mn含量增加对C扩散起到抑制作用。分子动力学模拟结果表明Mn含量增加会降低C原子扩散能力。Abstract: In order to study the decarburization effect of Fe-C-Mn alloy strips during solid-state decarburization and the diffusion effect of Mn content on C atoms, the solid-state decarburization test was carried out with Fe-2.7%C-(5%, 12%) Mn alloy composition and 1 mm thickness strips. The molecular dynamics simulation test was carried out by using Lammps software to explore the qualitative law of Mn content on C atom diffusion. The results show that the diffusion activation energy calculated by molecular dynamics simulation is basically the same as that calculated by solid state decarburization experiment. The diffusion activation energy of C atom in 5%Mn at 1223 K, 1323 K and 1363 K is 78.549 kJ·mol−1 and 83.805 kJ·mol−1, respectively. The solid-state decarburization test was carried out at 1363 K. The main limiting step of 5%Mn and 12%Mn alloy strips for 5~20 min was internal carbon diffusion. The decarburization effect of 12%Mn was not as good as that of 5%Mn, indicating that the increase of Mn content inhibited C diffusion. Molecular dynamics simulation results shows that the increase of Mn content reduces the diffusion ability of C atoms.
-
Key words:
- solid-state steelmaking /
- gas-solid reaction /
- molecular dynamics /
- diffusion coefficient
-
0. 引言
高炉转炉长流程工艺经过不断地改善优化,因高效低成本优点被钢铁企业广泛采用至今。其中,转炉吹氧脱碳处理会使钢液中含氧量升高,为降低氧含量,需进行必要的炉外精炼脱氧处理,然而钢液即使经过处理仍含有氧化物夹杂,影响钢坯质量。为此人们提出了一种铁水不经过转炉吹氧脱碳而直接将高炉铁水凝固成钢板薄带进行固态脱碳炼钢流程的新方法[1−2]。这种方法相比于高炉-转炉长流程炼钢,在保证金属不被大量氧化前提下,利用氧化性气氛将固态钢板薄带中碳脱除,极大地缩短了冶炼时间,降低了冶炼过程生产成本,提升了生产效率。目前,有大量学者对固态脱碳炼钢工艺[3−5]进行了探索研究,其中大量Fe-C合金固态脱碳[6−9]研究表明,Fe-C合金在脱碳过程中表面脱碳反应和薄带内部碳原子向界面的扩散为脱碳过程前中期限速环节;程荣[10]从Fe-C合金初始碳含量、脱碳温度、脱碳时间三个方面开展研究,确定了碳的扩散是限制脱碳反应的重要环节;侯耀斌[11]针对Fe-C合金探讨不同脱碳条件对脱碳效果的影响,研究建立了脱碳机理模型并分析脱碳过程,为固态脱碳试验条件和机理分析提供借鉴。碳的内部扩散是固态脱碳过程的重要环节之一,对固态脱碳效果起到关键作用,而C原子在Fe-C-Mn合金体系下扩散行为在试验和模拟两方面的相关研究较少,因此笔者利用分子动力学模拟手段对Fe-C-Mn合金体系开展C原子扩散行为影响及Mn含量对C原子扩散行为定性规律研究。
1. 试验条件及方法
1.1 模拟模型的建立
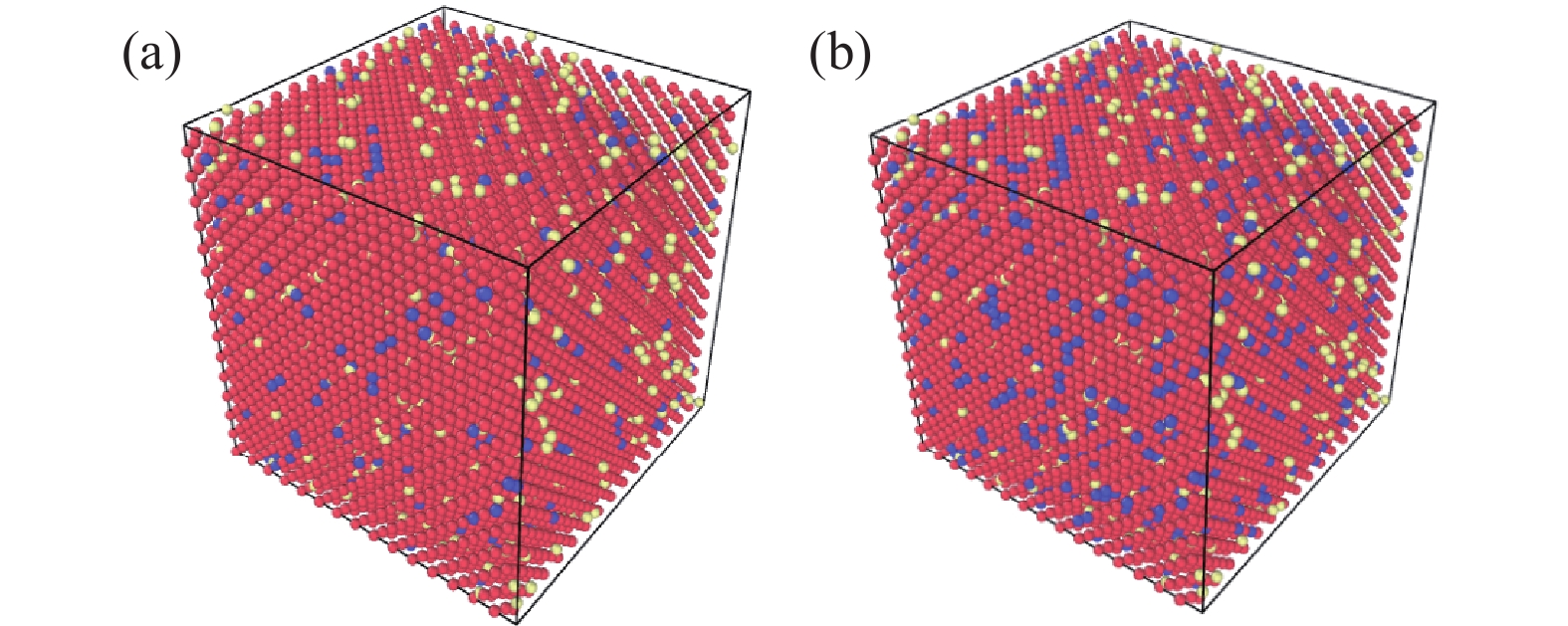
首先,在Lammps中建立15a0$ \times $15a0$ \times $15a0大小的Fcc结构的纯Fe超晶胞模型,a0(0.357 nm)为Fe的晶格常数,Fe原子总数为
13500 个,Mn原子按照比例随机置换Fe原子,随机插入2.7%C原子(即1701 个C原子),以铁为基体的合金中Mn、C原子是根据摩尔质量换算为对应比例的个数进行模拟建模,模型如图1所示,红色原子为Fe原子,紫色原子为Mn原子,黄色原子为C原子。分子动力学的基础为经典牛顿力学,微观原子间一直不停运动,彼此略微远离时相互吸引,过于靠近时又相互排斥。模型中原子数量越多,模拟结果越准确,但受限于计算水平,选择周期性边界条件,选择多体势函数(Mixed Element Atomistic Method,MEAM)的Fe-C[12],Fe-Mn[13]合金势能函数并通过LJ势函数[14]来描述C、Mn原子之间的相互作用;在等温等压(NPT)系综下,设置初始温度300 K,待体系能量稳定后,以1×1014 K/s升温速率从300 K升温至所设目标温度,弛豫
250000 步,时间步长为0.001 ps,在等温等体积(NVT)系综下运行500000 步计算C原子的均方位移(Mean Squared Displacement,MSD),用以表征原子扩散行为,并根据MSD计算出C原子的扩散系数。均方位移和扩散系数的表达式[15]如式(1)(2)所示。
$$ {\text{MSD}}(t) = \frac{1}{N} < \sum\limits_{t = 1}^N {{{[{r_i}(t) - {r_i}(0)]}^2}} > $$ (1) $$ {D_{self}} = \mathop {\lim }\limits_{t \to \infty } \frac{1}{{6Nt}} < \sum\limits_{i = 1}^N {{{[{r_i}(t) - {r_i}(0)]}^2}} > $$ (2) 式中, Dself为粒子的自扩散系数;N为粒子总数;t为时间;ri(t)和ri(0)分别为粒子在t和t0时刻的位置;由式(1)和式(2)可以得到自扩散系数和均方位移关系式如式(3)所示。
$$ {D_{self}} = \mathop {\lim }\limits_{t \to \infty } \frac{1}{{6t}}{\text{MSD}} $$ (3) 由式(3)可知,当t趋于无穷,均方位移和扩散系数线性相关,可根据其斜率求得扩散系数。
1.2 固态脱碳试验方法
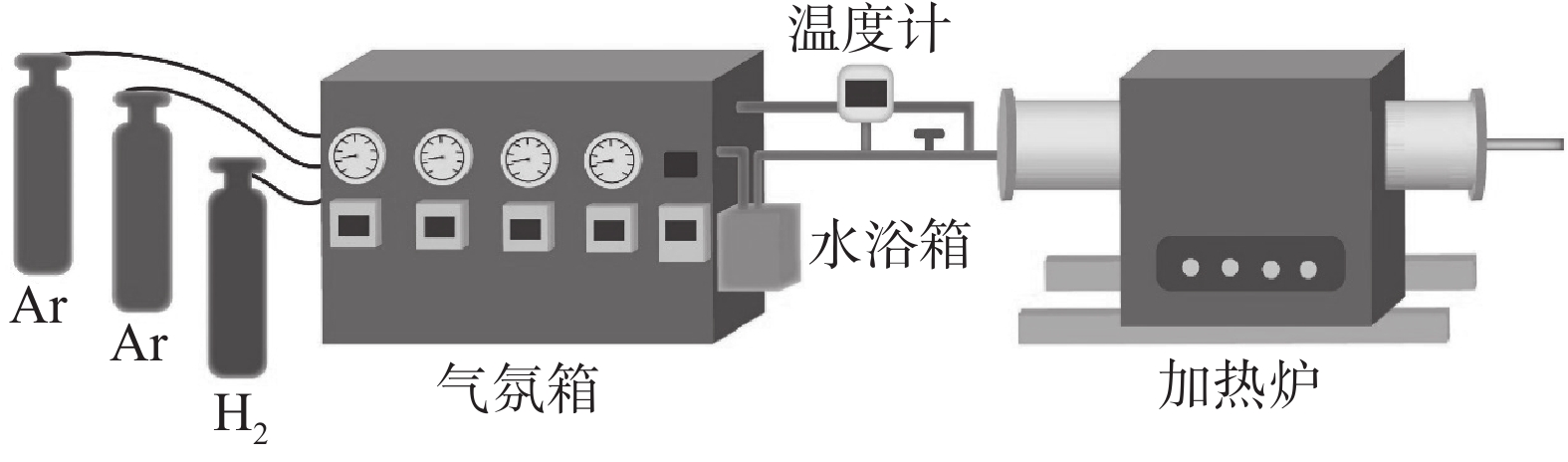
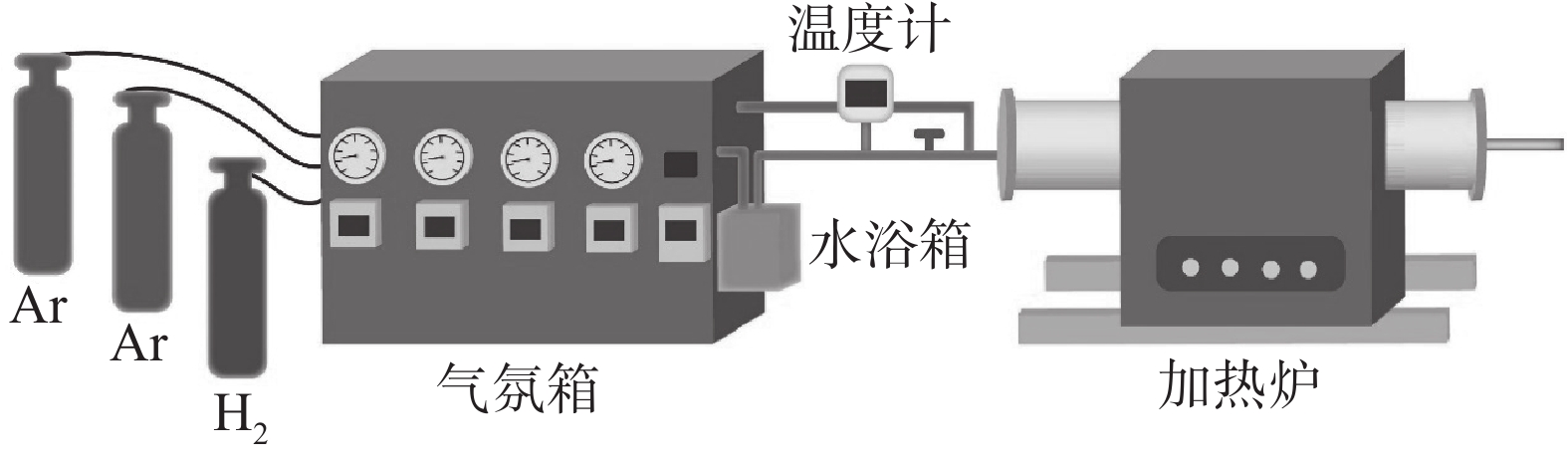
为探究固定Mn含量不同脱碳温度和固定温度不同Mn含量对脱碳效果的影响,以Fe-2.7%C-(5% 和12%)Mn成分合金薄带为研究对象,在Ar-H2O-H2气氛下进行脱碳试验,固态脱碳过程通过控制$P_{\mathrm{H}_2 \mathrm{O}} / P_{\mathrm{H}_2} $调控合金薄带表面氧化情况,以达到快速脱碳目的,图2为设备示意。
将尺寸规格为65 mm×55 mm×1 mm的合金薄带试样放入加热炉中,脱碳前将加热炉反复抽真空三次,加热过程中通入Ar气保护气体,以防止加热过程中合金薄带氧化。达到脱碳反应温度后,通过调整水浴露点温度调控脱碳气氛$P_{\mathrm{H}_2 \mathrm{O}} / P_{\mathrm{H}_2} $[16−17],脱碳试验条件依据前期研究成果选定[18−19],通入Ar和H2气混合气体,气体总流量为
1500 mL/min,其中H2占总气氛流量的20%,$P_{\mathrm{H}_2 \mathrm{O}} / P_{\mathrm{H}_2} $=0.56,设置不同时间进行断点脱碳试验。2. 讨论
2.1 温度对C扩散行为影响
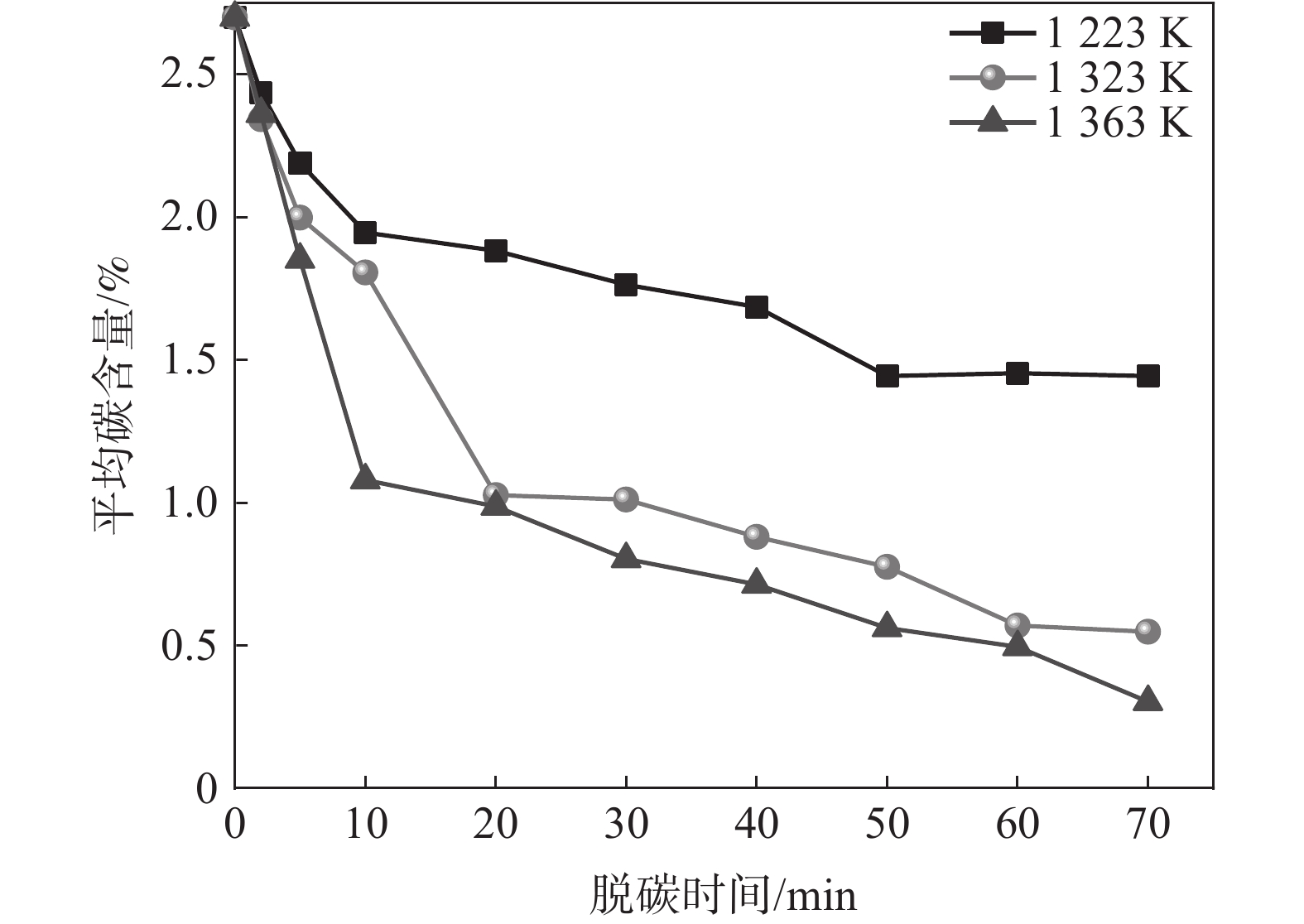
为了探究温度对Fe-C-Mn合金薄带脱碳效果的影响,在
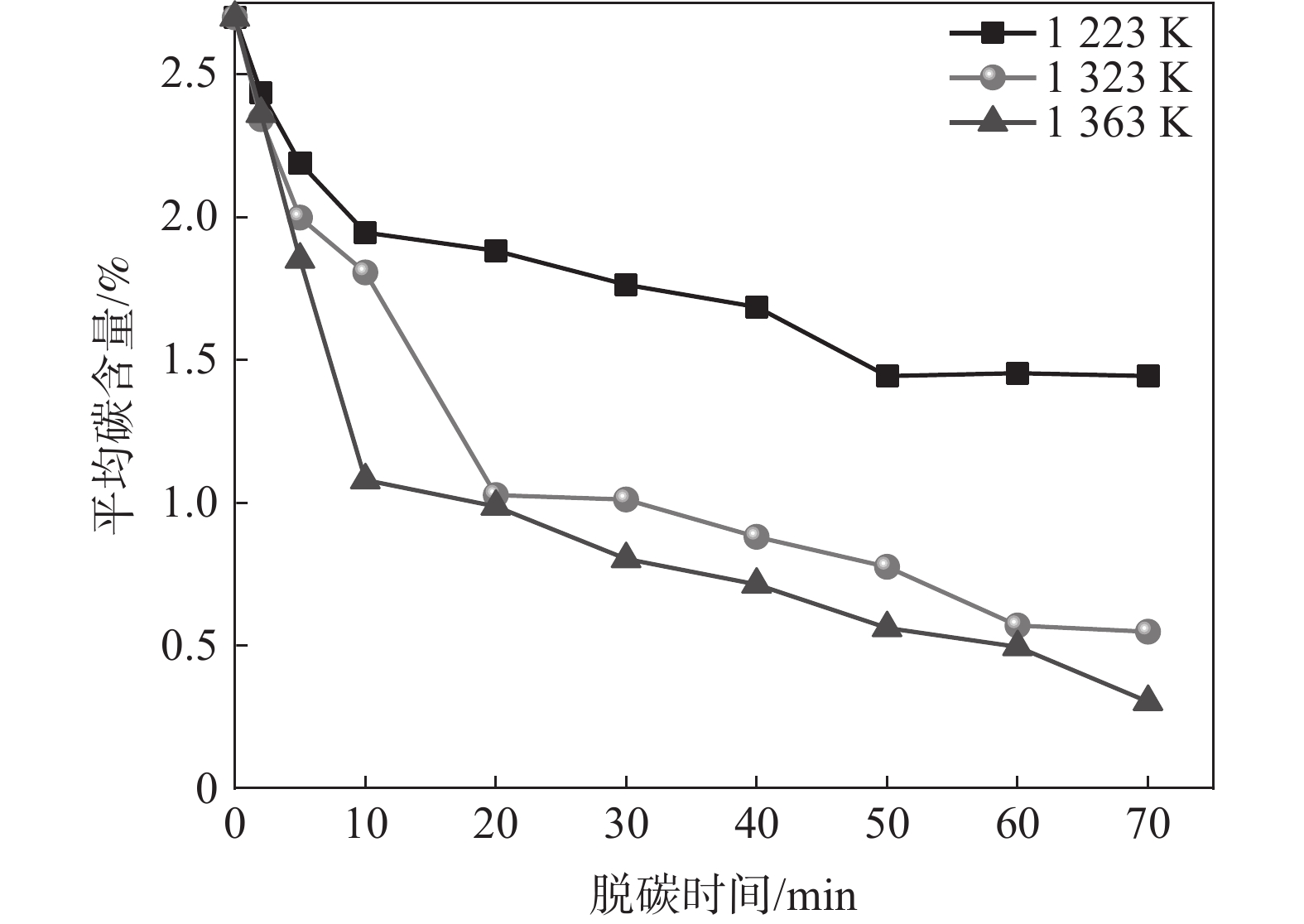
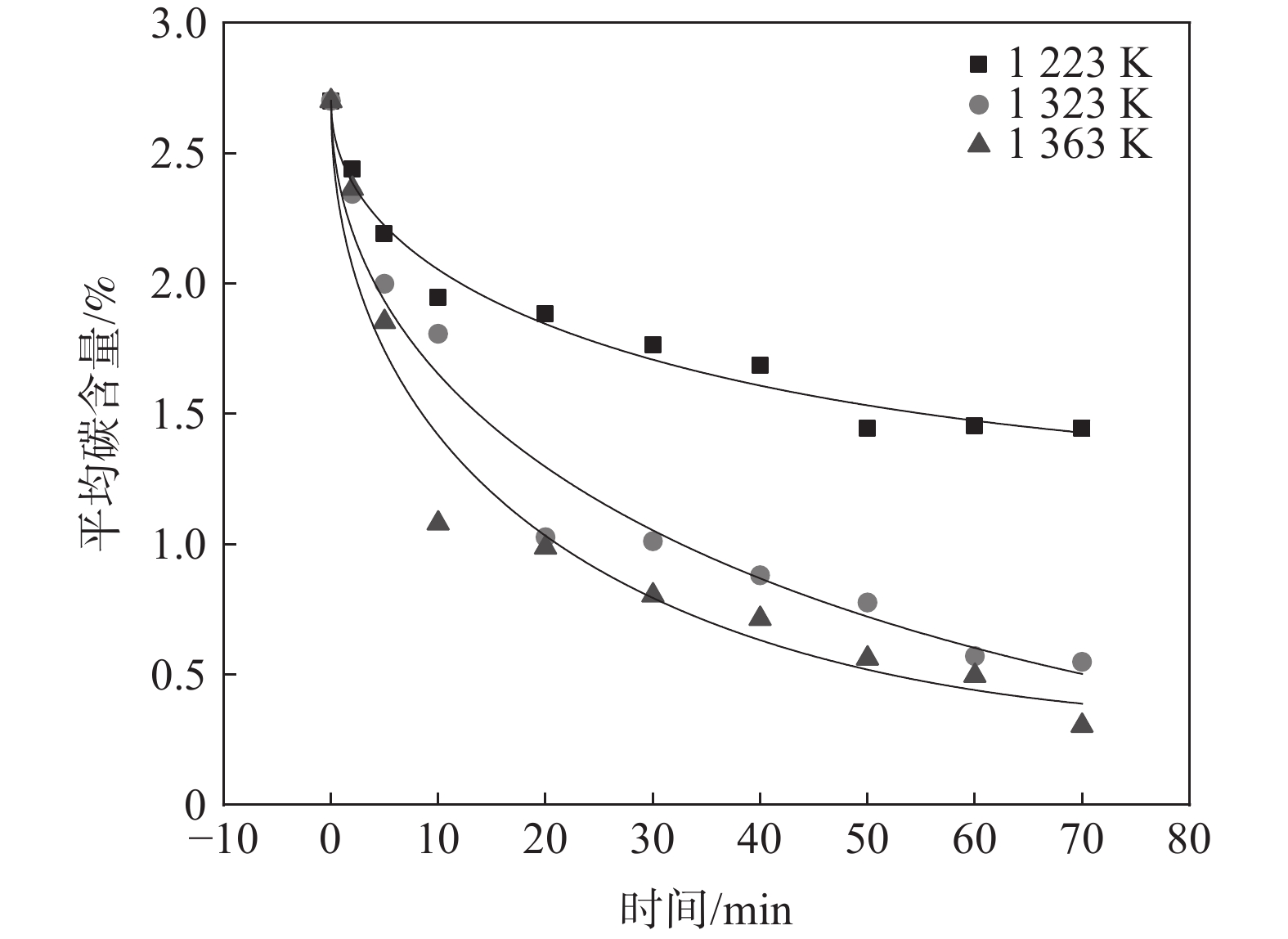
1223 、1323 K和1363 K下开展断点试验研究,脱碳时间为0~70 min,试验结果如图3所示。由图3可知,随着脱碳温度的提高,脱碳效果改善显著。脱碳时间增加,不同脱碳温度下薄带平均碳含量均逐渐降低,脱碳速率逐渐趋于平缓。当温度由
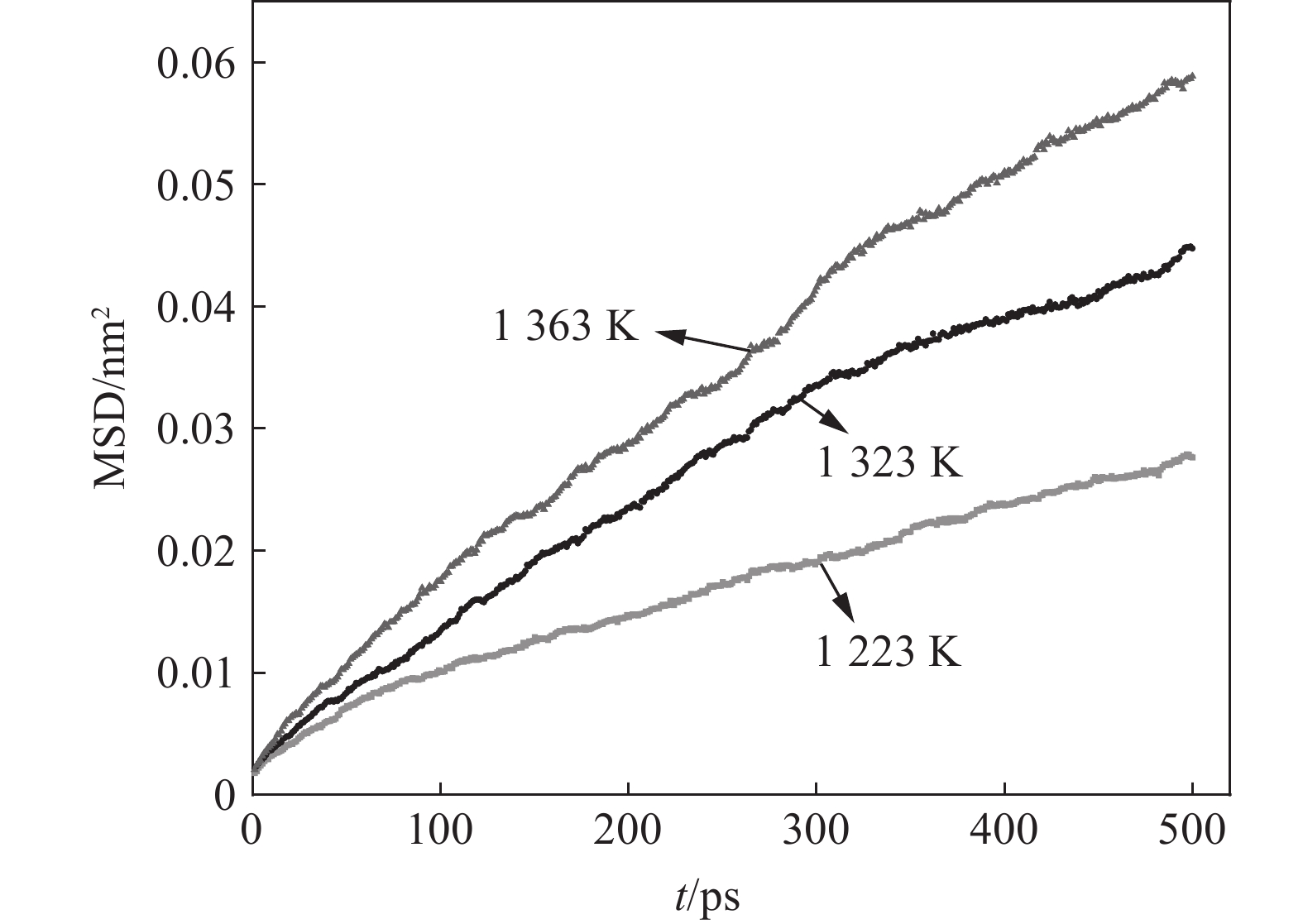
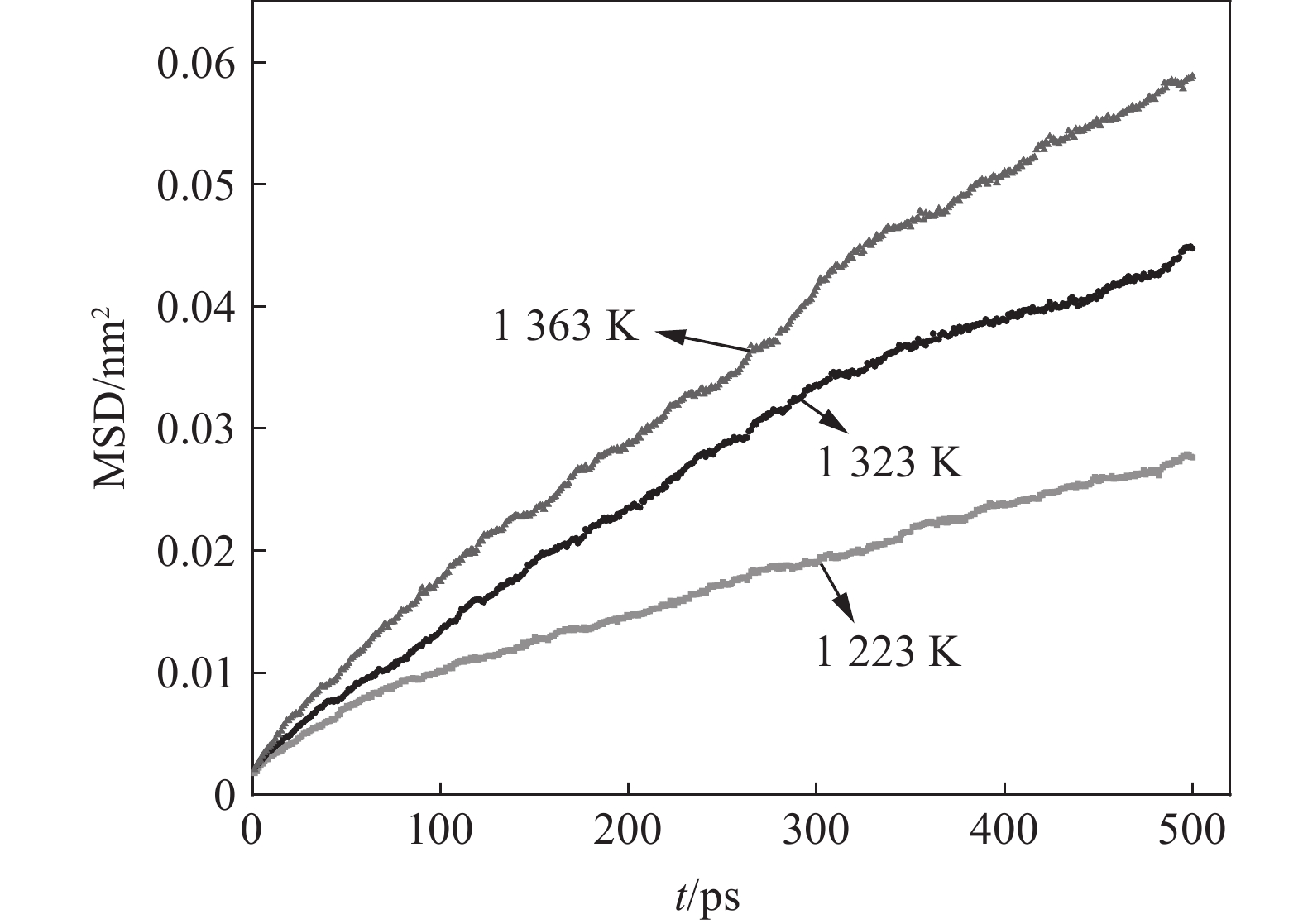
1223 K提高至1323 K时,脱碳效果尤为明显,20 min时1323 K下薄带的平均碳含量明显低于1223 K。从脱碳动力学角度分析,温度升高导致合金薄带扩散能力增强,为脱碳过程持续不断提供固溶碳源,因此,1323 K下平均碳含量降低明显。而1323 K与1363 K温差较小,两者脱碳效果差别并不显著。为从微观原子角度探索温度对Fe-C-Mn体系中C扩散行为影响,进行分子动力学模拟试验,研究C原子在不同温度下MSD位移曲线随时间的变化,模拟结果如图4所示。
由图4可知,C原子MSD随时间的延长不断增加,温度提高C原子扩散能力增强,这一结果与脱碳效果规律一致。
为验证脱碳结果和模拟结果结合的合理性,分别从脱碳动力学与分子动力学模拟两方面计算C原子扩散激活能。侯耀斌等人[11]开展了Fe-C合金脱碳试验,并提出了脱碳模型机理,当合金薄带内碳扩散为整个脱碳过程中的限制性环节时,可以利用菲克第一定律和质量衡算进行简化分析,碳含量的变化可以用式(4)近似表达。
$$ {[\% C]_t} = {[\% C]_0} - k{t^{1/2}}{\text{ + }}k't $$ (4) 式中,[%C]0为初始碳含量;t为时间;k,k'为系数,其值分别为:
$$ \begin{split} &k = \frac{{2(a + b)}}{{ab}}{k_1}{k_2} =\\& \frac{{2(a + b)}}{{ab}} \sqrt {2\frac{D}{\rho }({C_2} - {C_3})\left[ {{C_1} - \frac{1}{2}({C_2} + {C_3})} \right]} \end{split} $$ (5) $$ k' = 4\frac{{{k_1}^2{k_2}}}{{ab}}{k_1}{k_2} = \frac{{8D}}{{ab\rho }}({C_2} - {C_3}) $$ (6) 式中,a,b分别为薄带截面宽度和厚度;ρ为密度;C1为薄带碳含量;C2为表面碳含量;C3为固溶“饱和碳含量”;D为扩散系数。
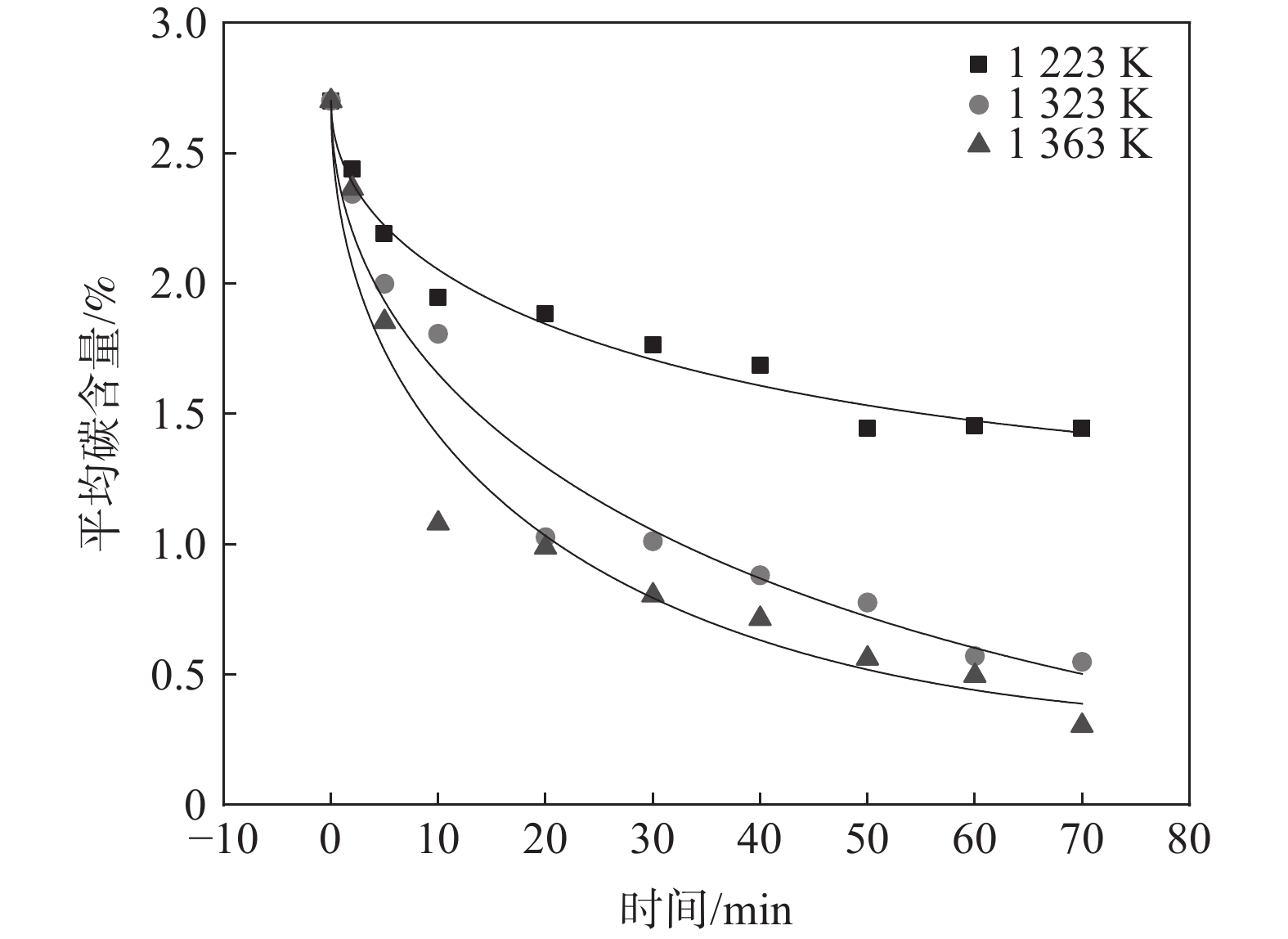
将图3脱碳数据根据式(4)进行数据拟合得到不同温度下脱碳数据拟合曲线,如图5所示。
根据式(4)脱碳动力学扩散机理模型可知,系数k正比于D1/2,k'正比于D,随着脱碳时间的延长,k'起到决定作用,因此使用k'近似代表D得到固态脱碳时C的扩散激活能。
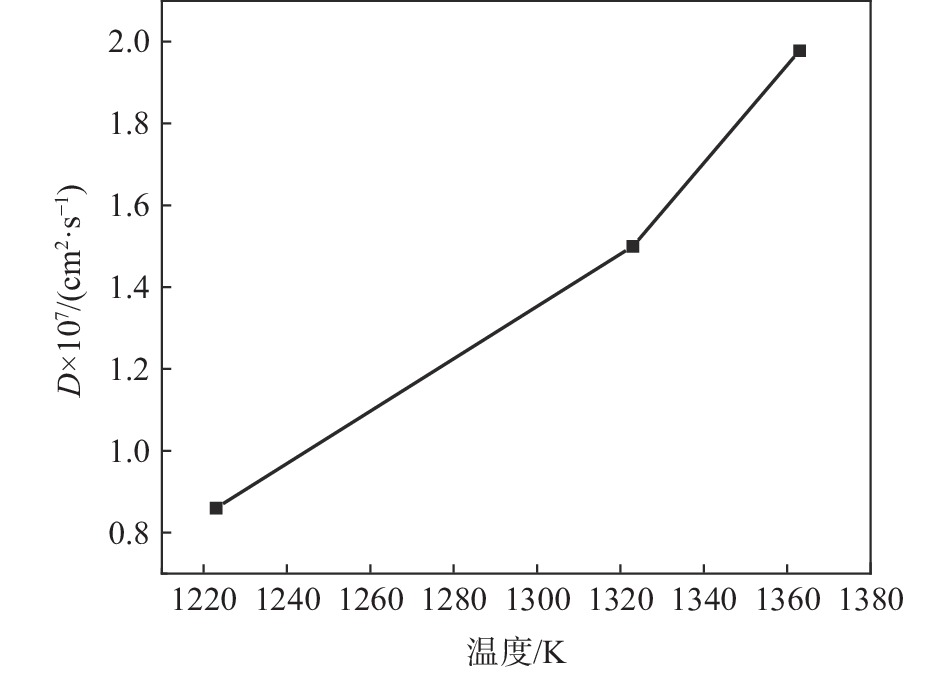
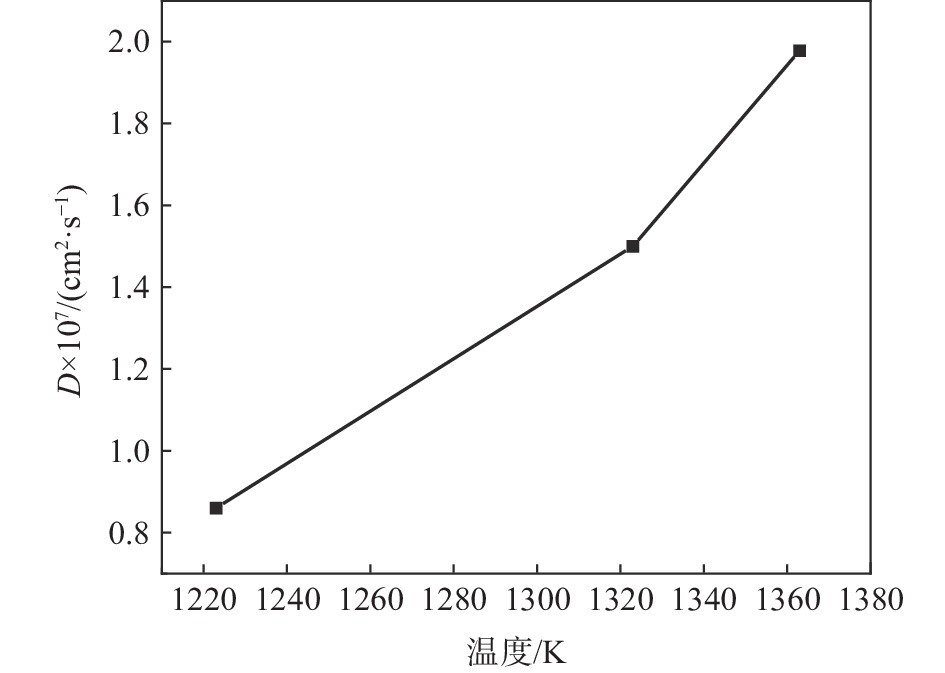
将图4按式(3)可以计算出不同温度下分子动力学模拟理论C原子扩散系数分别为8.6×10−8、1.5×10−7、1.978×10−7cm2/s,并绘制了扩散系数随温度变化曲线,如图6所示。
由原子扩散系数表达式(7)可知,扩散系数D与温度T成指数关系,随着温度增加,扩散系数不断增大。通过式(7)计算C原子扩散激活能。
$$ D = {D_0}\exp\left(\frac{{ - Q}}{{RT}}\right) $$ (7) 式中,D为扩散系数;D0为扩散常数;Q为扩散激活能;R扩散系数常数;T为温度。
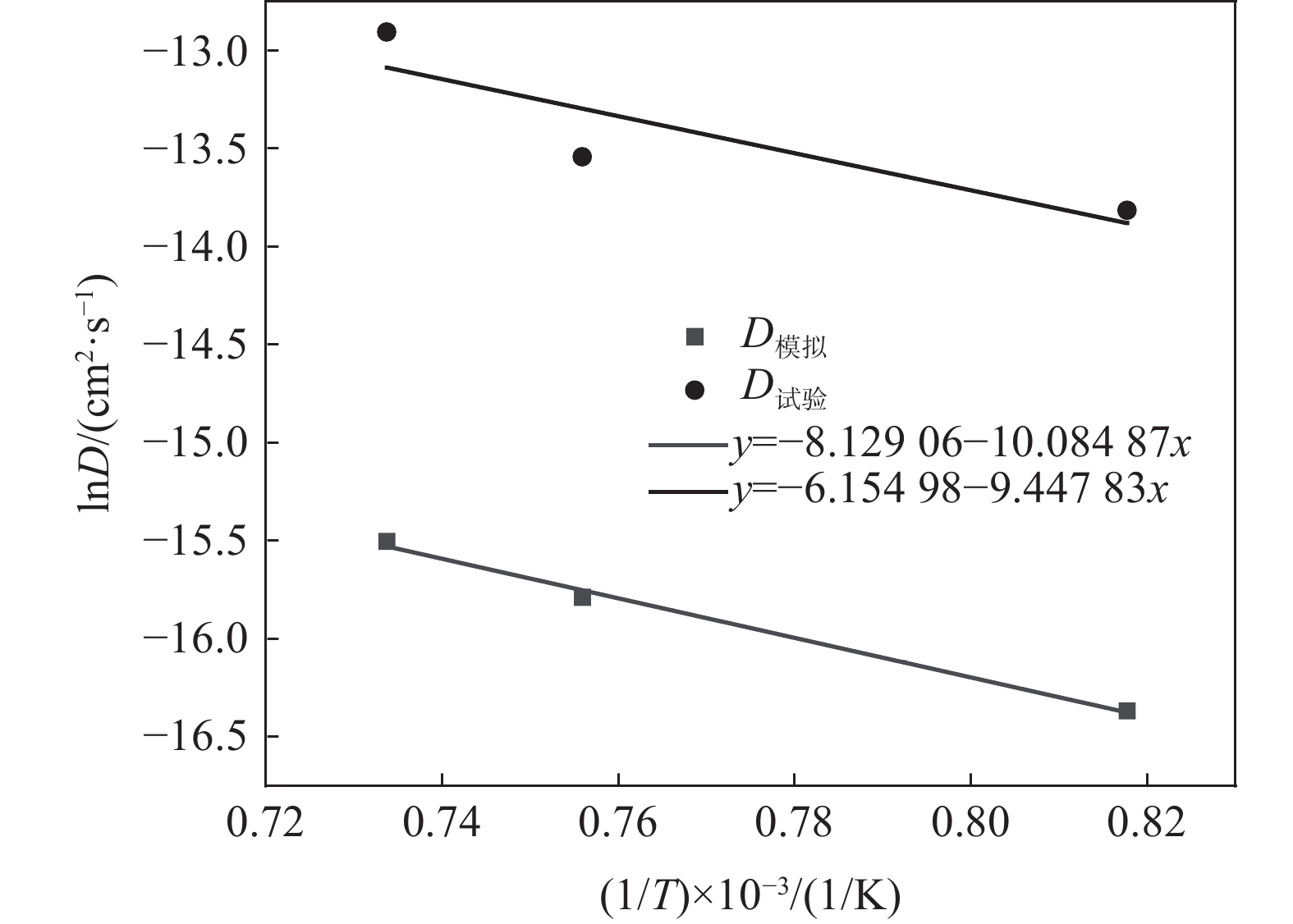
将式(7)两边取对数,可得线性表达式(8)。
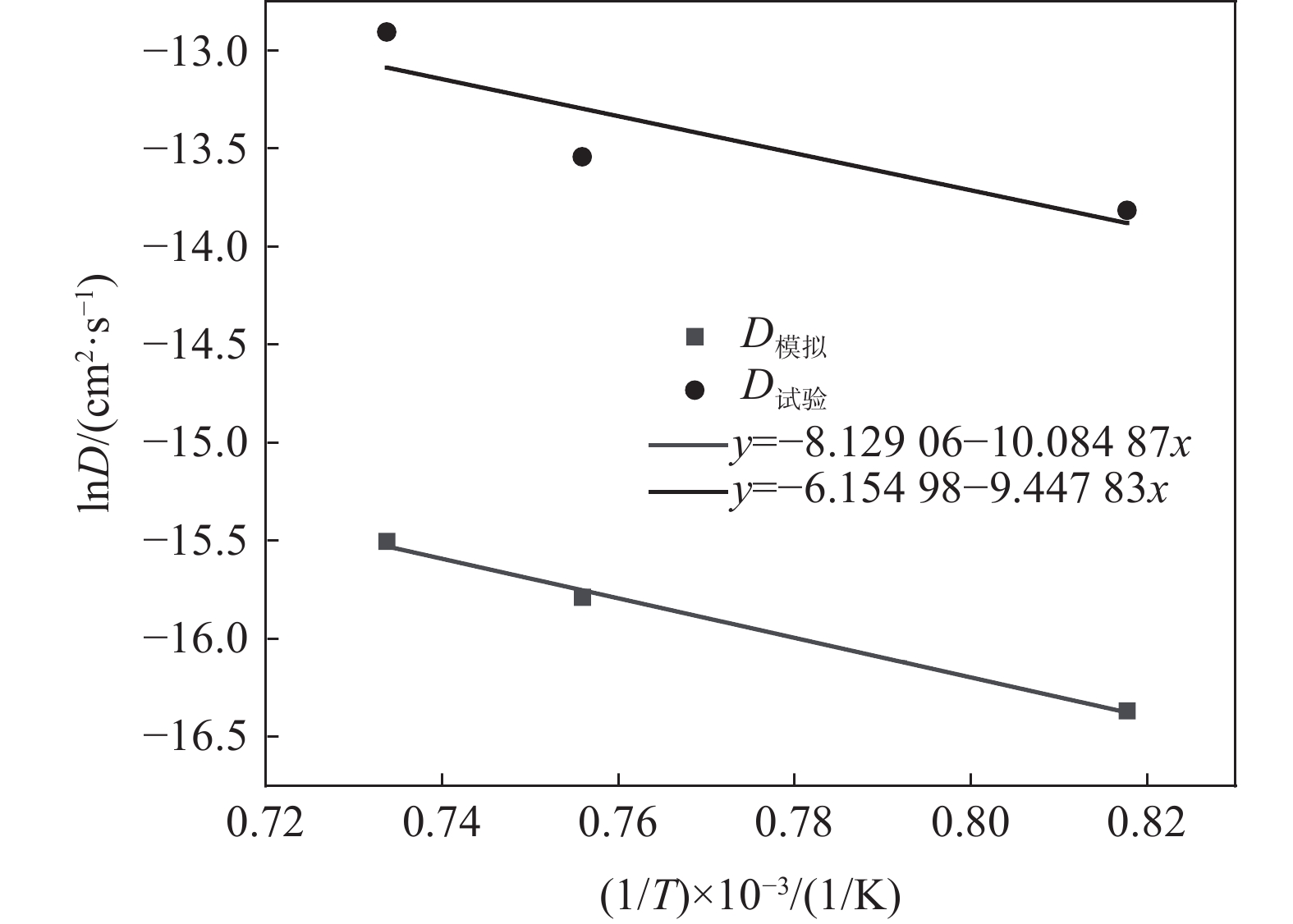
$$ \ln D = \ln {D_0} - \frac{Q}{R}\frac{1}{T} $$ (8) 如图7所示,对lnD与温度的倒数1/T成线性关系进行拟合,通过拟合斜率(−Q/R)求得扩散激活能Q,根据截距lnD0求得扩散系数常数D0,从而得到以下完整表达式:
$$ \begin{split} &{D}_{模拟}=2.948\times {10}^{-4}\mathrm{exp}\;\left(\frac{-83\;805}{RT}\right)\;\;{{\mathrm{cm}}}^{2}/{{\mathrm{s}}}\\ & Q=83.805\;{{\mathrm{kJ/mol}}} \end{split} $$ $$ \begin{split} &{D}_{试验}=1.39\times {10}^{-4}\mathrm{exp}\;\left(\frac{-78\;549}{RT}\right)\;\;{{\mathrm{cm}}}^{2}/{{\mathrm{s}}}\\ & Q=78.549\;{\mathrm{kJ/mo{l}}} \end{split} $$ 通过固态脱碳试验和分子动力学模拟试验分别得到了两者的扩散激活能,可见两者结果基本一致,分子动力学模拟结果可信。
2.2 Mn含量对C扩散行为影响
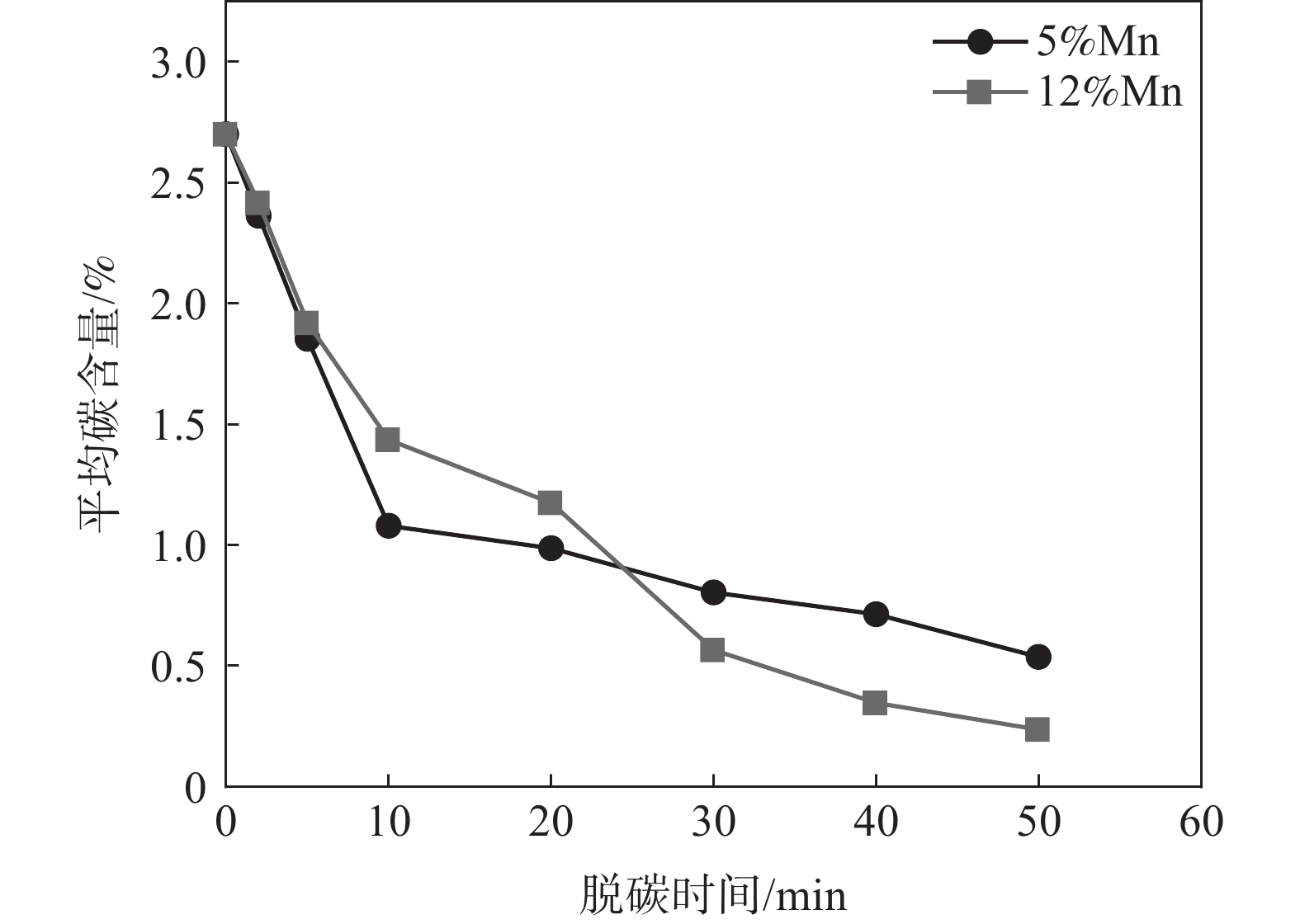
L Král[20]等研究表明,C在奥氏体中扩散能力随着Mn含量的增加而减弱。为探究Mn含量(5%和12%)对脱碳效果的影响作用开展固态脱碳试验,固态脱碳温度设为
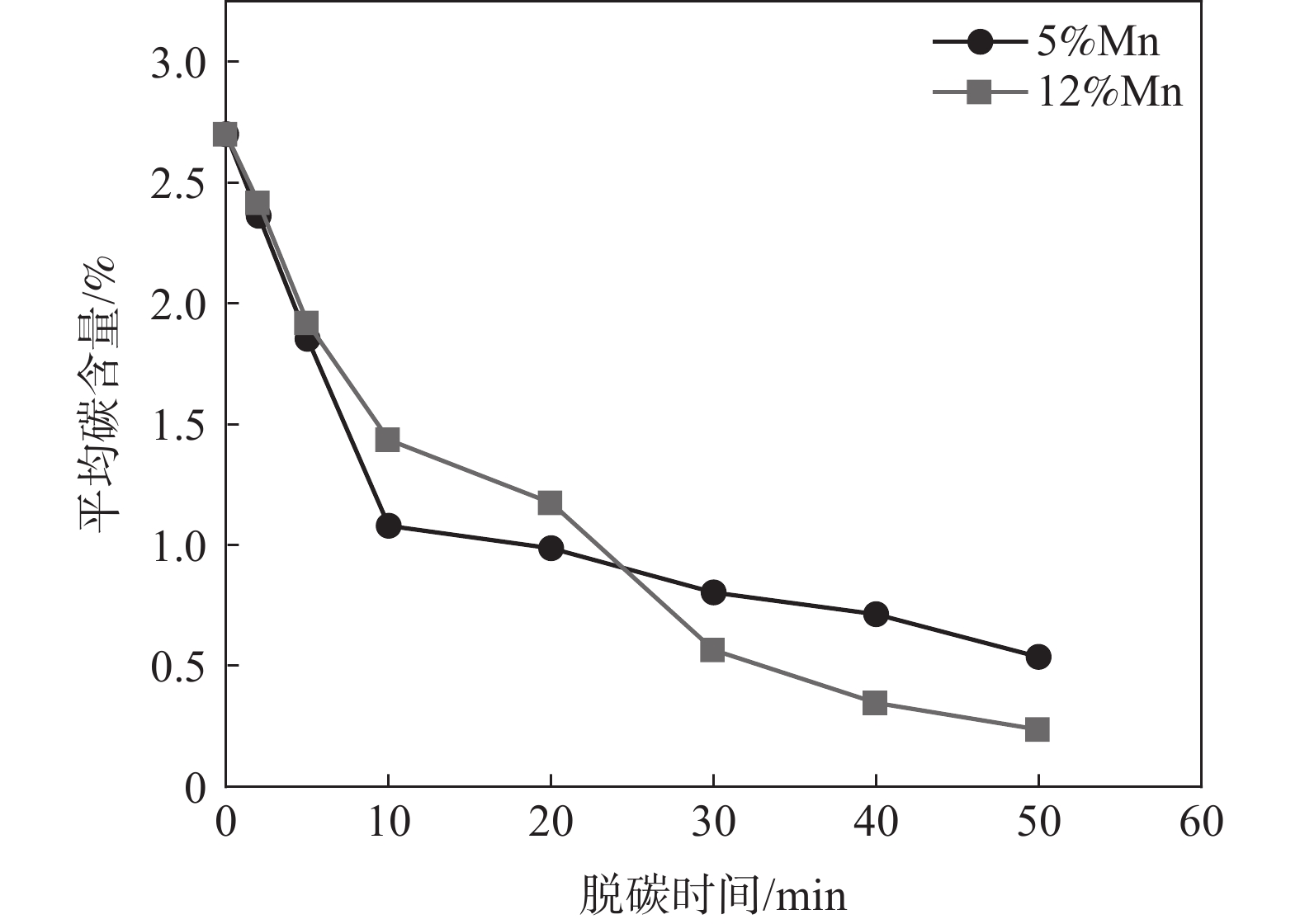
1363 K,气体总流量和$P_{\mathrm{H}_2 \mathrm{O}} / P_{\mathrm{H}_2} $保持不变,脱碳时间设置为0~50 min。固态脱碳后薄带平均碳含量随脱碳时间的变化如图8所示。由图8可知,20 min后12%Mn平均碳含量低于5%Mn,说明脱碳的限制性环节发生了转变。5~20 min主要限制性环节是内部碳的扩散,20~30 min为薄带内C扩散向渗碳体分解转化;30 min后,由于高锰薄带含有较多的渗碳体,渗碳体分解能力较高,可为薄带提供更多固溶碳进行脱碳反应,保证了中后期脱碳高于低锰时的脱碳效果,薄带平均碳含量因此而降低。利用分子动力学模拟在
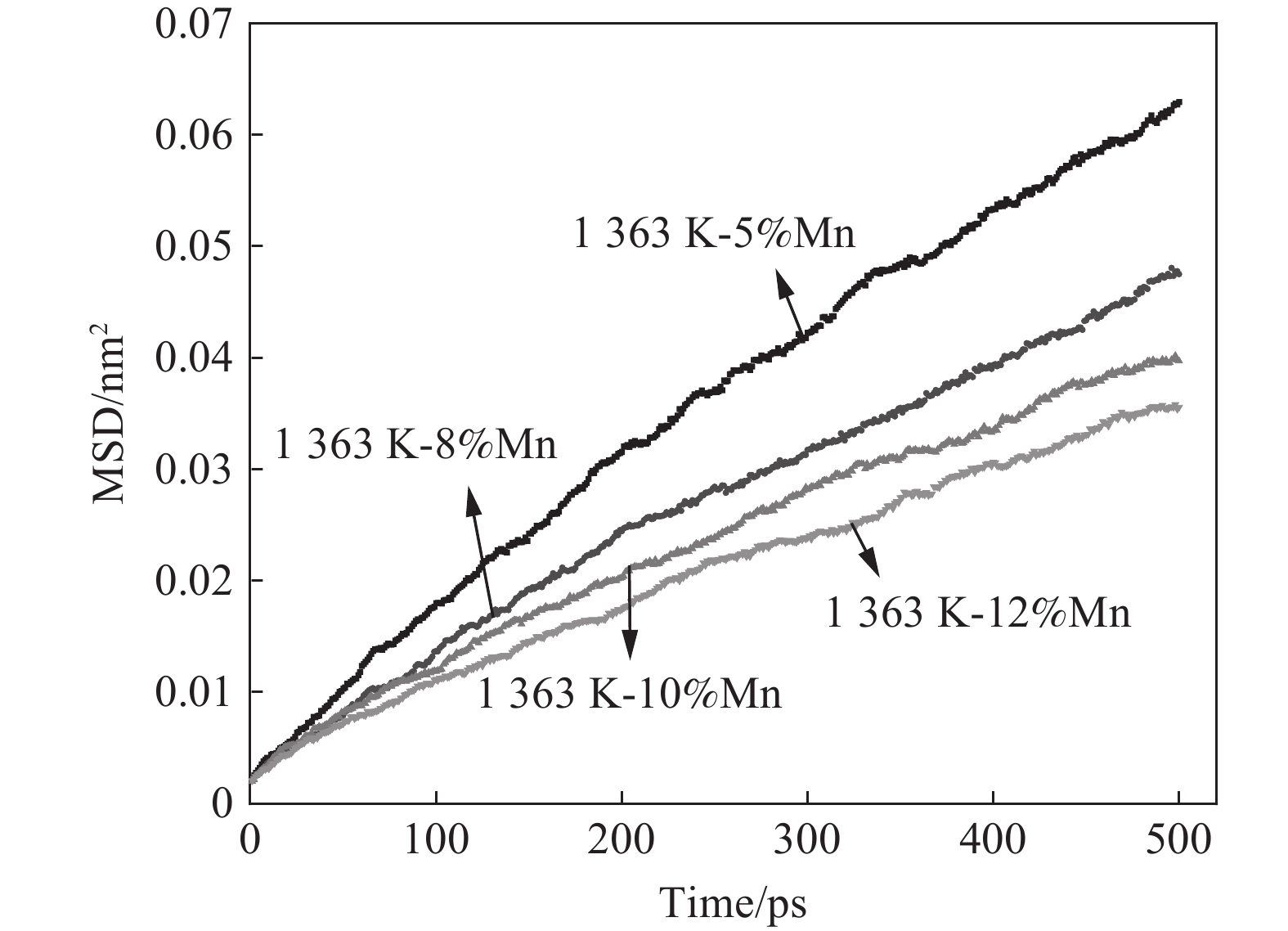
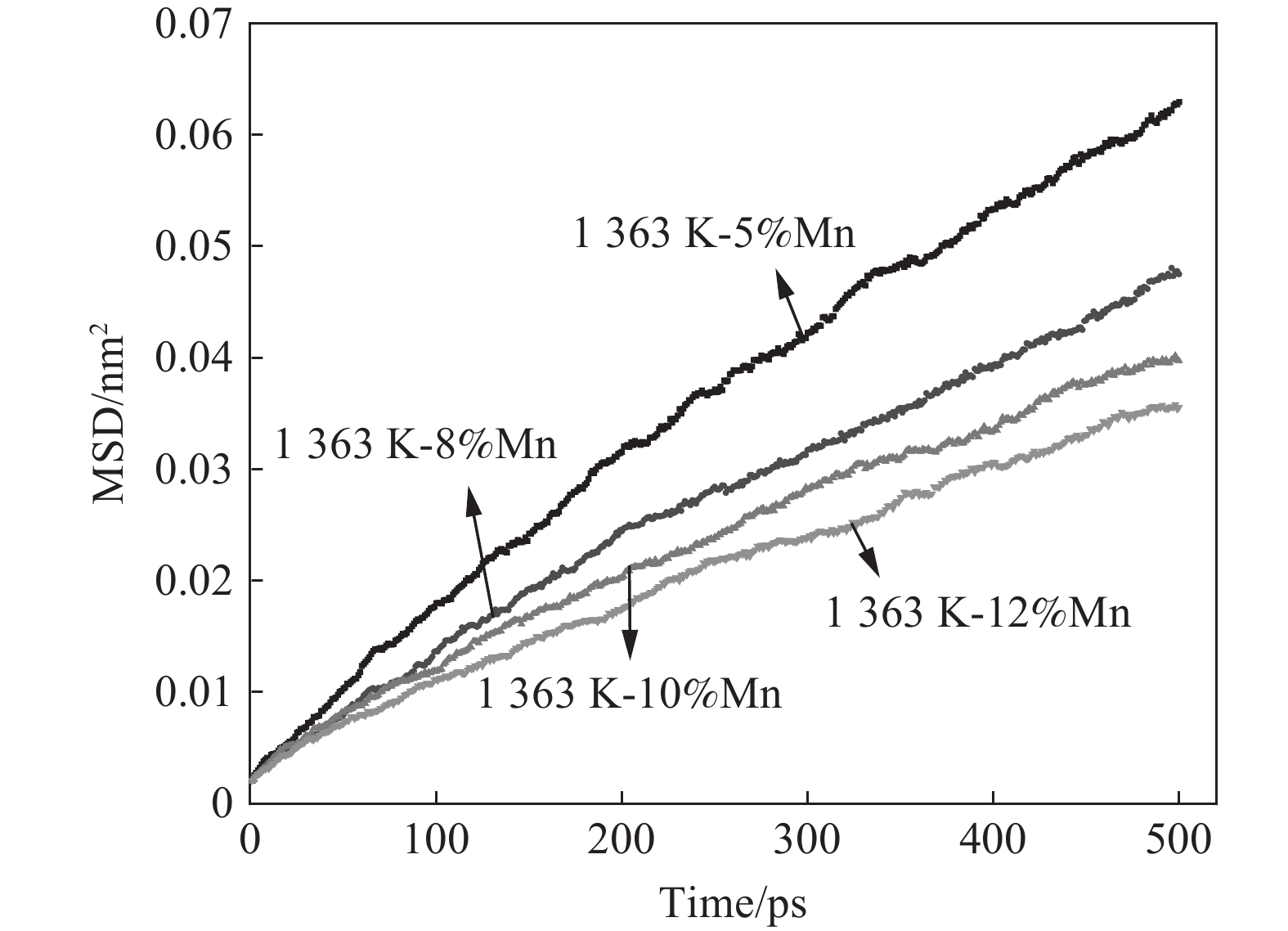
1363 K温度下进行Fe-(5%和12%)Mn-2.7%C模拟研究并计算扩散系数。图9为不同Mn含量随时间变化曲线。由图9可知,增加Mn含量对C扩散能力具有抑制作用,同图8所示不同,Mn含量前20 min脱碳效果趋势一致。由图9计算得到5%Mn和12%Mn在
1363 K温度下扩散系数分别为1.978×10−7、 1.08×10−7 cm2/s。为排除模拟结果的偶然性,在Fe-C-Mn合金体系中增加8%Mn和10%Mn含量模拟测试,如图9所示,可看到Mn含量的增加对Fe-C-Mn合金体系中C原子扩散行为起到抑制作用。3. 结论
1) 对Fe-5%Mn-2.7%C合金成分薄带进行固态脱碳试验,结果表明,随着温度的升高,脱碳效果显著。分子动力学模拟理论结果显示,C原子扩散能力随着温度的提高不断增强。固态脱碳动力学机理模型和分子动力学模拟理论计算的扩散激活能分别为78.549 kJ/mol和83.805 kJ/mol,两者结果基本一致。
2) 在
1363 K温度下对5%Mn和12%Mn合金薄带进行固态脱碳试验,结果表明5~20 min时主要限制性环节为碳的内部扩散,12%Mn平均碳含量高于5%Mn,说明Mn含量的增加对碳的内部扩散起到抑制作用,30 min 后由于渗碳体分解,12%Mn薄带脱碳效果提升。分子动力学模拟显示,随着模拟体系中Mn含量的增加,C原子扩散能力不断减弱。 -
-
[1] Li Yaqiang, Ai Liqun. The proposal and progress of new process research on solid-state steelmaking[J]. Foundry Technology, 2017,38(1):8-11. (李亚强, 艾利群. 固态炼钢新工艺研究的提出与进展[J]. 铸造技术, 2017,38(1):8-11.Li Yaqiang, Ai Liqun. The proposal and progress of new process research on solid-state steelmaking[J]. Foundry Technology, 2017, 38(1): 8-11. [2] Hong Lukuo, Ai Liqun. New process of solid steelmaking[J]. Industrial Metrology, 2015,25(S1):161-163. (洪陆阔, 艾立群. 浅谈固态炼钢新工艺[J]. 工业计量, 2015,25(S1):161-163.Hong Lukuo, Ai Liqun. New process of solid steelmaking[J]. Industrial Metrology, 2015, 25(S1): 161-163. [3] Park J O, Long T V, Sasaki Y. Feasibility of solid-state steelmaking from cast iron decarburization of rapidly solidified cast iron[J]. ISIJ International, 2012,98(5):151-160. [4] Lee W H, Park J O, Lee J S, et al. Solid state steelmaking by decarburisation of rapidly solidified high carbon iron sheet[J]. Ironmaking & Steelmaking, 2012,39(7):530-534. [5] Sharif S E, Mirjalili M, Khaki J V. A new approach in solid state steelmaking from thin cast iron sheets through decarburization in CaCO3 pack[J]. ISIJ International, 2018,58(10):1791-1800. doi: 10.2355/isijinternational.ISIJINT-2018-250 [6] Sun Caijiao, Ai Liqun, Hong Lukuo, et al. Decarburization kinetics of iron-carbon alloy ribbons by gas-solid reaction in Ar-CO-CO2 atmosphere[J]. Materials Reports, 2021,35(24):24142-24146. (孙彩娇, 艾立群, 洪陆阔, 等. Ar-CO-CO2气氛下铁碳合金薄带气固反应脱碳动力学研究[J]. 材料导报, 2021,35(24):24142-24146.Sun Caijiao, Ai Liqun, Hong Lukuo, et al. Decarburization kinetics of iron-carbon alloy ribbons by gas-solid reaction in Ar-CO-CO2 atmosphere[J]. Materials Reports, 2021, 35(24): 24142-24146. [7] Sun Caijiao, Ai Liqun, Hong Lukuo, et al. Decarburization mechanism of Fe-C alloy in H2 / H2O atmosphere by gas-solid reaction[J]. Materials Reports, 2020,34(20):20112-20117. (孙彩娇, 艾立群, 洪陆阔, 等. H2/H2O气氛下Fe-C合金气固反应脱碳机理[J]. 材料导报, 2020,34(20):20112-20117.Sun Caijiao, Ai Liqun, Hong Lukuo, et al. Decarburization mechanism of Fe-C alloy in H2 / H2O atmosphere by gas-solid reaction[J]. Materials Reports, 2020, 34(20): 20112-20117. [8] Chen Pengfei, Ai Liqun. Study on gas-solid reaction decarburization of medium carbon domain iron-carbon alloy ribbons[J]. Iron Steel Vanadium Titanium, 2020,41(3):105-109. (陈鹏飞, 艾立群. 中碳域铁碳合金薄带气—固反应脱碳研究[J]. 钢铁钒钛, 2020,41(3):105-109.Chen Pengfei, Ai Liqun. Study on gas-solid reaction decarburization of medium carbon domain iron-carbon alloy ribbons[J]. Iron Steel Vanadium Titanium, 2020, 41(3): 105-109. [9] Hong Lukuo. Temperature and atmosphere control of gas-solid reaction decarburization of iron-carbon alloy ribbons[D]. Tangshan: North China University of Science and Technology, 2015. (洪陆阔. 铁碳合金薄带气—固反应脱碳温度与气氛控制[D]. 唐山:华北理工大学2015.Hong Lukuo. Temperature and atmosphere control of gas-solid reaction decarburization of iron-carbon alloy ribbons[D]. Tangshan: North China University of Science and Technology, 2015. [10] Cheng Rong. Kinetic analysis of gas-solid reaction decarburization of iron-carbon alloy ribbons[D]. Tangshan: North China University of Science and Technology, 2016. (程荣. 铁碳合金薄带气—固反应脱碳动力学分析[D]. 唐山:华北理工大学, 2016.Cheng Rong. Kinetic analysis of gas-solid reaction decarburization of iron-carbon alloy ribbons[D]. Tangshan: North China University of Science and Technology, 2016. [11] Hou Yaobin. Decarburization of Fe-C alloy by step heating in CO-CO2 atmosphere[D]. Tangshan: North China University of Science and Technology, 2021. (侯耀斌. CO-CO2气氛下分段加热对Fe-C合金脱碳研究[D]. 唐山:华北理工大学, 2021.Hou Yaobin. Decarburization of Fe-C alloy by step heating in CO-CO2 atmosphere[D]. Tangshan: North China University of Science and Technology, 2021. [12] Lee B J. A modified embedded-atom method interatomic potential for the Fe–C system[J]. Acta Materialia, 2006,54(3):701-711. doi: 10.1016/j.actamat.2005.09.034 [13] Kim Y M, Shin Y H, Lee B J. Modified embedded-atom method interatomic potentials for pure Mn and the Fe–Mn system[J]. Acta Materialia, 2009,57(2):474-482. doi: 10.1016/j.actamat.2008.09.031 [14] Rappé A K, Casewit C J, Colwell K S, et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations[J]. Journal of the American Chemical Society, 1992,114(25):10024-10035. doi: 10.1021/ja00051a040 [15] Wang Shaogang, Liu Cuixia, Jian Zengyun. Molecular dynamics simulation of diffusion coefficient of Al-Cu alloy[J]. Journal of Xi'an Technological University, 2018,38(6):559-564. (王少刚, 刘翠霞, 坚增运. Al-Cu合金扩散系数的分子动力学模拟研究[J]. 西安工业大学学报, 2018,38(6):559-564.Wang Shaogang, Liu Cuixia, Jian Zengyun. Molecular dynamics simulation of diffusion coefficient of Al-Cu alloy[J]. Journal of Xi'an Technological University, 2018, 38(6): 559-564. [16] Li Yaqiang, Ai Liqun, Li Qiang, et al. 1 mm iron-carbon alloy strip gas-solid reaction decarburization test[J]. Iron & Steel, 2017,52(5):19-23, 35. (李亚强, 艾立群, 李强, 等. 1 mm铁碳合金薄带气—固反应脱碳试验[J]. 钢铁, 2017,52(5):19-23, 35.Li Yaqiang, Ai Liqun, Li Qiang, et al. 1 mm iron-carbon alloy strip gas-solid reaction decarburization test[J]. Iron & Steel, 2017, 52(5): 19-23, 35. [17] Li Yaqiang. Effect of atmosphere conditions on gas-solid reaction decarburization of iron-carbon alloy ribbons[D]. Tangshan: North China University of Science and Technology, 2017. (李亚强. 气氛条件对铁碳合金薄带气—固反应脱碳的影响[D]. 唐山:华北理工大学, 2017.Li Yaqiang. Effect of atmosphere conditions on gas-solid reaction decarburization of iron-carbon alloy ribbons[D]. Tangshan: North China University of Science and Technology, 2017. [18] Meng Fanjun, Ai Liqun, Hong Lukuo, et al. Experimental study on decarburization of Fe-C-Mn ribbons in Ar-H2O-H2 atmosphere[J]. Iron Steel Vanadium Titanium, 2021,42(5):132-137. (孟凡峻, 艾立群, 洪陆阔, 等. Ar-H2O-H2气氛下Fe-C-Mn薄带脱碳试验研究[J]. 钢铁钒钛, 2021,42(5):132-137.Meng Fanjun, Ai Liqun, Hong Lukuo, et al. Experimental study on decarburization of Fe-C-Mn ribbons in Ar-H2O-H2 atmosphere[J]. Iron Steel Vanadium Titanium, 2021, 42(5): 132-137. [19] Sun Caijiao, Ai Liqun, Hong Lukuo, et al. Study on solid state steelmaking from thin cast iron sheets through decarburization in H2O-H2[J]. Ironmaking & Steelmaking, 2020,47(9):1015-1021. [20] Král L, Million B, Čermák J. Diffusion of carbon and manganese in Fe-C-Mn[C]//Defect and Diffusion Forum. Trans Tech Publications Ltd, 2007, 263: 153-158. -






 下载:
下载:








 下载:
下载: