Research on preparation and OER properties of vanadium doped cobalt iron layered double hydroxide
-
摘要: 开发环境友好且毒性相对较低的析氧反应(OER)电催化剂是目前水分解的最大困难之一。采用电沉积的方法在泡沫镍(NF)上原位生长了钴铁层状双氢氧化物(CoFe LDH)和钒掺杂的钴铁层状双氢氧化物(V-CoFe LDH)纳米片,并将其作为OER催化剂以探究其析氧性能。研究结果表明,在碱性介质中,当电流密度为100 mA·cm−2时,CoFe LDH和V-CoFe LDH的过电位分别为396 mV和356 mV,CoFe LDH和V-CoFe LDH分别具有224 mV·dec−1和210 mV·dec−1的Tafel斜率。此外,相比于CoFe LDH,V-CoFe LDH电催化剂具有大的电化学比表面积和优异的电解液润湿性。这些结果均表明V的引入有助于增强材料的OER性能。结合密度泛函理论计算和试验结果证明,V的掺杂不仅优化了材料的电子结构,增强了导电性,同样降低了吸附能,增强了催化剂与电解液的接触。Abstract: Exploiting environmentally friendly and relatively low-toxicity oxygen evolution reaction (OER) electrocatalysts is currently one of the biggest difficulties in water splitting. In this work, cobalt iron layered double hydroxide (CoFe LDH) and vanadium-doped cobalt iron layered double hydroxide (V-CoFe LDH) nanosheets are in situ grown on nickel foam (NF) by electrodeposition as an effective OER catalyst. When CoFe LDH and V-CoFe LDH samples are used as electrocatalysts, they both exhibit excellent OER performance. In an alkaline media, when the current density is 100 mA·cm−2, the overpotentials of CoFe LDH and V-CoFe LDH are small overpotentials of 396 mV and 356 mV, respectively. Tafel slopes of CoFe LDH and V-CoFe LDH are 224 mV·dec−1 and 210 mV·dec−1, respectively. The electrochemical activity specific surface area of V-CoFe LDH electrocatalysts is higher than CoFe LDH electrocatalysts. Moreover, the V-CoFe LDH electrocatalyst has better wetting properties for the electrolyte. These all indicate that the introduction of V helps to enhance the OER performance of the material. Density functional theory calculations and experimental results have shown that the doping of V not only optimizes the electronic structure of the material, enhances conductivity, but also reduces adsorption energy and enhances the contact between the catalyst and electrolyte. This work demonstrates that V-CoFe LDH is a highly promising OER electrocatalyst.
-
0. 引言
随着全球能源问题日益突出,氢能作为一种可再生、清洁和高效率的能源受到广泛关注。利用风能、太阳能等可再生能源来进行电解水制氢,是目前公认的一种切实可行的方法[1−3]。电解水过程主要包含两个半反应过程:负极的析氢反应(HER)和正极的析氧反应(OER)。众所周知,析氧反应(OER)涉及复杂的四电子转移过程,电化学能垒较高,动力学较低,使得析氧过程电位远高于水的理论分解电压(1.23 V),经常需要电催化剂来降低高活化势垒[4],因此找到具有优异OER催化性能的电催化剂是电解水制氢领域面临的最大挑战之一。为解决这一问题,研究人员开展了高效OER催化剂的相关研究。
目前,常见的OER催化剂主要为以Ir/Ru等贵金属及其氧化物为典型代表[5−6],但是它们在酸性或者碱性电解液高阳极电位下均具有差的稳定性,同时,其稀缺性和价格昂贵的特点限制了大规模应用[7−8]。因此寻找贵金属催化剂的替代品成为研究者关注的核心问题。因层状双过渡金属氢氧化物(LDH)资源丰富、廉价易得且高效,被认为是极具潜力的OER催化剂,近年来备受研究人员青睐[9]。层状双过渡金属氢氧化物的d/f电子轨道特性使它们在OER反应中表现出出色的电化学性能[10]。此外,由于不同过渡金属元素的协同作用有利于降低氢氧化物的带隙,从而提高催化剂的导电性,进一步提高其催化性能[11−12]。目前研究较多的双金属LDH催化剂有NiFe LDH、NiCo LDH和 CoFe LDH等[13],然而,大多数层状双过渡金属氢氧化物都是以团聚颗粒的形式获得的,比表面积有限、电子导电性差、稳定性差[14]。为了满足实际应用的要求,提高电催化活性,研究人员通过各种方式来改善其有限的活性表面积,提升导电性和稳定性。清华大学石教授课题组[15]采用环境友好的电化学方法合成负载有NiFe LDH纳米片石墨网络结构的电极,该催化剂的电催化性优于贵金属IrO2催化,由于NiFe LDH和石墨的协同作用,以及这种3D的网络结构能够有效增大催化剂的电化学活性面积,进而有效促进OER反应过程的电荷传递。此外,也有研究人员通过掺杂其他元素来调节LDH催化剂的电子结构,以增强OER性能。目前,已被报道表明三价的Ce、Cr、Mn掺杂在LDH结构中能调节LDH电子结构,提升该材料的OER性能[16−17]。
CoFe LDH材料具有独特的二维层状结构、丰富多变的化学组成、高分散的金属阳离子、优异的稳定性和成本低廉等优点,在OER反应中有广泛的应用前景,但不良的导电性和有限的活性位点阻碍了CoFe LDH的工业化应用。钒元素因其丰富的d轨道电子、丰富的价态、高的电子导电性及富集的活性位点,有表现出独特的OER电化学特性的潜力。若将V元素引入到CoFe LDH中制备出V掺杂的钴铁水滑石材料,将有望获得析氧性能更为优异的电催化剂[18]。
基于以上,设计并制备出了一种利用引入钒元素掺杂调控钴铁水滑石氧析出性能的高效催化剂。通过XRD、FTIR、UV-vis等表征手段对钒掺杂前后钴铁水滑石的物相和结构信息进行表征。结合DFT和电化学测试结果,系统地解释了钒掺杂钴铁水滑石(V-CoFe LDH)结构与OER性能间的构效关系。不仅展现了一种高效率的OER催化剂制备方法,而且提供了一种利用钒等过渡金属元素来进行电子结构调控以促进电化学性能提升的研发思路。
1. 试验部分
试验中使用的所有化学品都是分析级的,无需进一步纯化即可使用。在这项工作中使用了电阻率为18.25 MΩ·cm的去离子水。
1.1 泡沬镍基底的前处理
在制备电极材料前,需要对泡沫镍(NF)基底进行一系列预处理,以便后续水滑石纳米片的生长。首先将裁成多块正方形的泡沫镍(1 cm×1 cm,极耳约0.3 cm × 0.3 cm,纯度99.8%)分别用无水乙醇和去离子水超声15 min,以除去泡沫镍基底表面残留的油污及杂质,随后用浓度为0.5 mol/L的HNO3超声处理10 min,以除去泡沫镍表面的氧化物以及碱性物质,接着用去离子水超声处理10 min,用吹风机吹干置于干净的表面皿中备用。
1.2 钴铁水滑石(CoFe LDH)电极的制备
CoFe LDH采用电沉积方法制备。先称取28.11 g的CoSO4·6H2O(99.5%,分析纯,上海麦克林生化科技有限公司)和20.201 g的Fe(NO3)3·9H2O(99%,分析纯,天津市大茂化学试剂有限公司)溶解于100 mL的去离子水中,Co和Fe的摩尔比为1:0.5,将溶液置于超声机中超声15 min,以保证溶质充分溶解,得到混合液备用。取20 mL混合液作为三电极体系的电解液,以饱和甘汞电极作为参比电极,1 cm×1 cm的Pt片电极为对电极,以经处理后的泡沫镍电极作为工作电极,初始电压为-1 V,电沉积时间为400 s,待电沉积完成后,取出工作电极,用去离子水进行多次冲洗,随后置于干净的表面皿上,在60 ℃下的真空干燥箱中放置12 h,泡沫镍表面沉积的材料即为钴铁水滑石(CoFe LDH)。
1.3 钒掺杂钴铁水滑石(V-CoFe LDH)电极的制备
V-CoFe LDH采用电沉积方法制备。先称取28.11 g的CoSO4·6H2O(99.5%,分析纯,上海麦克林生化科技有限公司)和 20.201 g的Fe(NO3)3·9H2O(99%,分析纯,天津市大茂化学试剂有限公司)和
1.9504 g的V2(SO4)3(99.9%,成都先进金属材料产业技术研究院股份有限公司)溶解于100 mL的去离子水中,Co,Fe和V的摩尔比为1∶0.5∶0.05,将溶液置于超声机中超声15 min,以保证溶质充分溶解,得到混合液备用。取20 mL混合液作为三电极体系的电解液,以饱和甘汞电极作为参比电极,1 cm×1 cm的Pt片电极为对电极,以经处理后的泡沫镍电极作为工作电极,初始电压为-1V,电沉积时间为400 s,待电沉积完成后,取出工作电极,用去离子水进行多次冲洗,随后置于干净的表面皿上,在60 ℃下的真空干燥箱中放置12 h,泡沫镍表面沉积的材料即为V掺杂钴铁水滑石(V-CoFe LDH)。1.4 物相和结构信息表征
利用X射线衍射仪对合成的材料进行晶型表征,参数具体为:扫描范围为3°~70°、测试条件为:Cu靶,Ka射线,电压为40 kV,电流为40 A。利用场发射扫描电镜(SEM)对样品进行微观形貌表征。利用EDS mapping对样品的元素种类、含量及分布进行分析。利用赛默飞-IS50傅里叶变换红外光谱仪(FT-TR)对样品的插层离子种类进行表征,波数范围为400~
4000 cm−1。使用水接触角测试仪测定接触角(Contact angle),即液-固交界线与气-液界面切线的夹角θ,当液相为电解液时,接触角可以作为宏观上判断材料对电解液的润湿性参数。1.5 电化学性能测试
采用上海辰华760E (CHI 760E, CH Instruments Inc., Chenghua, Shanghai) 电化学工作站。将试验制备的电极直接作为工作电极(工作电极固定工作面积为1 cm2),Pt片电极作为对电极,饱和甘汞电极作为参比电极,测试使用的电解液为1 mol/L 的KOH溶液。
1.5.1 线性扫描伏安法测试(LSV)
LSV是对电极体系施加一个线性变化的电压,通过记录工作电极响应电流随电极电位变化的关系曲线,通过 LSV 曲线可以得到电催化氧化过程的过电位(Overpential,η),催化剂的η计算方法如公式(1)所示。测试条件为:电压范围为 0~1 V,扫描速率为10 mV·s−1, LSV 测试不进行IR补偿。
$$ \begin{split} &\eta(\mathrm{mV})=[\text { measured value }(\text { vs. SCE })+0.241+\\ & 0.059\;2 \mathrm{pH}-1.229(\text { vs. RHE })] \times 1\;000\\[-40pt] \end{split} $$ (1) 1.5.2 电化学阻抗(EIS)测试
EIS是通过施加一个频率不同的小振幅正弦波电位测得交流电压与电流的比值随正弦波频率的变化曲线。测试参数如下:频率区间为 10−2~105 Hz,振幅为 5 mV,测试电位(vs SCE) 0.5 V 。
1.5.3 循环伏安测试(CV)
循环伏安法是在电极上先施加一个随时间变化的电压,当达到所需的测试范围时再反向施加电压,记录电流随电位变化的曲线。试验主要通过利用不同扫速下的 CV 曲线比较电催化剂的电化学活性面积(ECSA)。
1.5.4 塔菲尔斜率(Tafel Slope)
Tafel斜率是基于方程(2)从没有IR补偿的LSV曲线导出的,其中b 代表了塔菲尔斜率;j代表电流密度(mA·cm−2) ;η代表过电位(mV)。
$$ \eta=b \log j+a $$ (2) 1.5.5 稳定性测试
稳定性测试是通过采用施加恒定电流观察电压随时间变化来评价催化剂的稳定性。采用了计时电压法进行稳定性测试。
2. 结果与讨论
2.1 CoFe LDH和V-CoFe LDH的形貌及结构表征
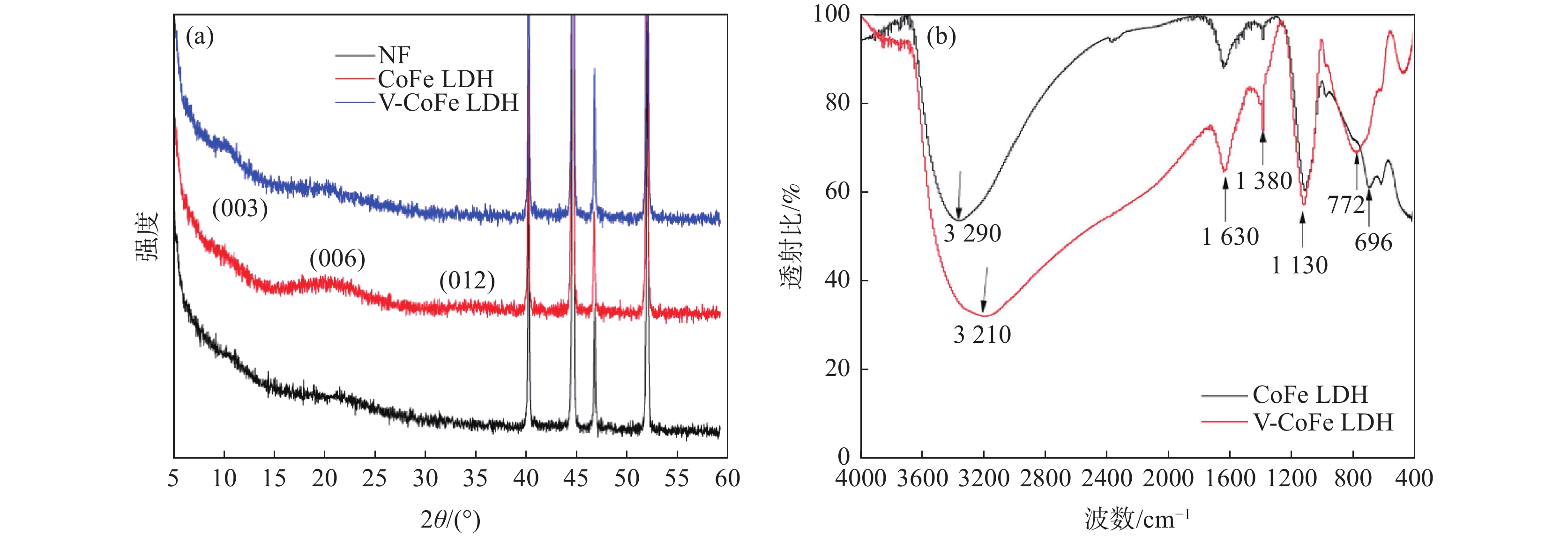
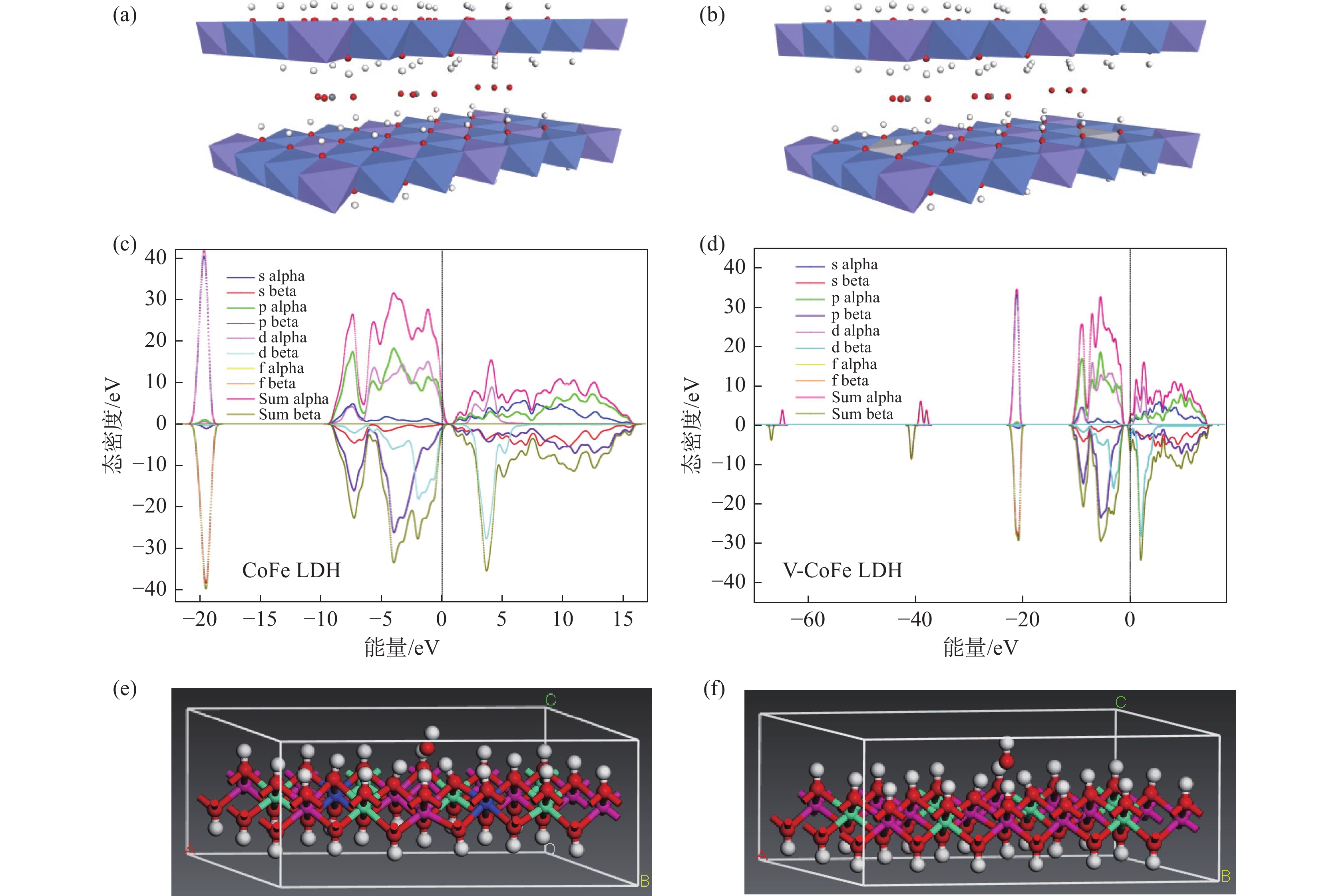
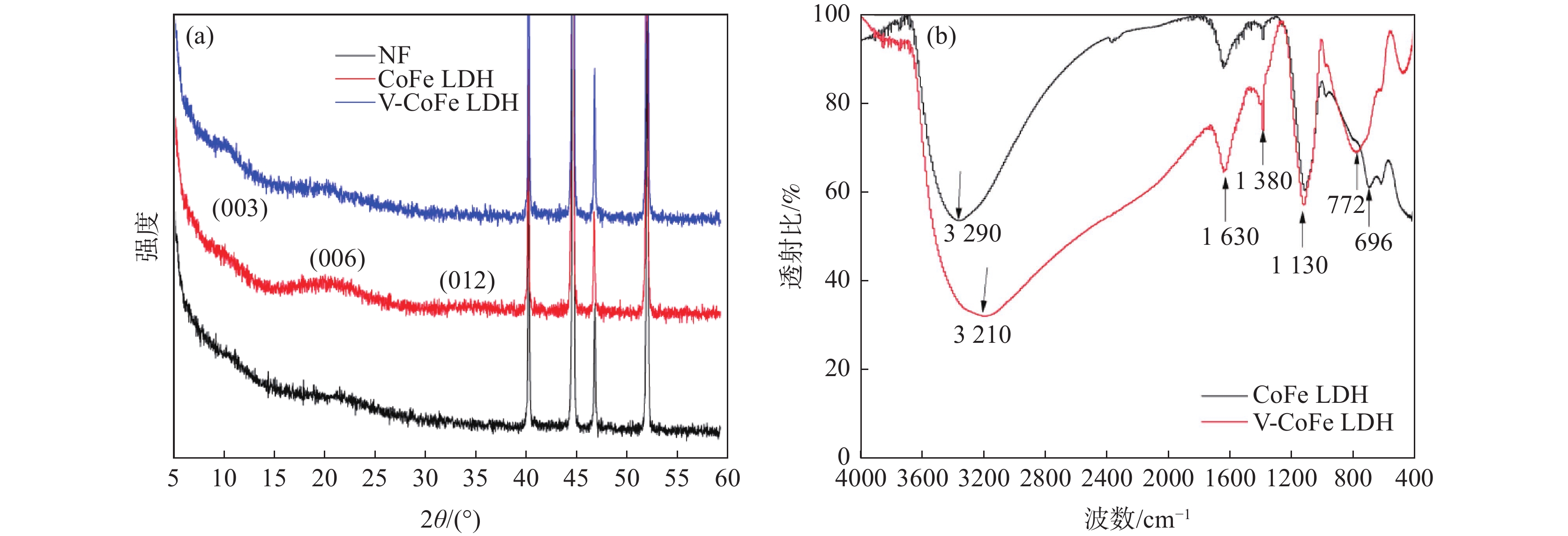
通过XRD对所制备的CoFe LDH和V-CoFe LDH进行表征。XRD结果如图1(a)所示,根据LDH的标准图谱JCPDS No.46-0605,所合成材料的2θ在10.1°,20.3°,33.6°位置分别出现了对应于(003)(006)(012)晶面的特征衍射峰,表现出水滑石典型的层状特征。值得说明的是,钒元素的掺杂并不影响钴铁水滑石的晶体结构,未出现其他物相的特征衍射峰。同时,d(003)=1.2 nm,说明水滑石层间阴离子为碳酸根,即我们所制备出的是碳酸根插层的钴铁和钒掺杂的钴铁水滑石[19−20]。为了进一步证明这一点,将所制备的样品进行FT-IR表征,结果如图1(b)所示,在
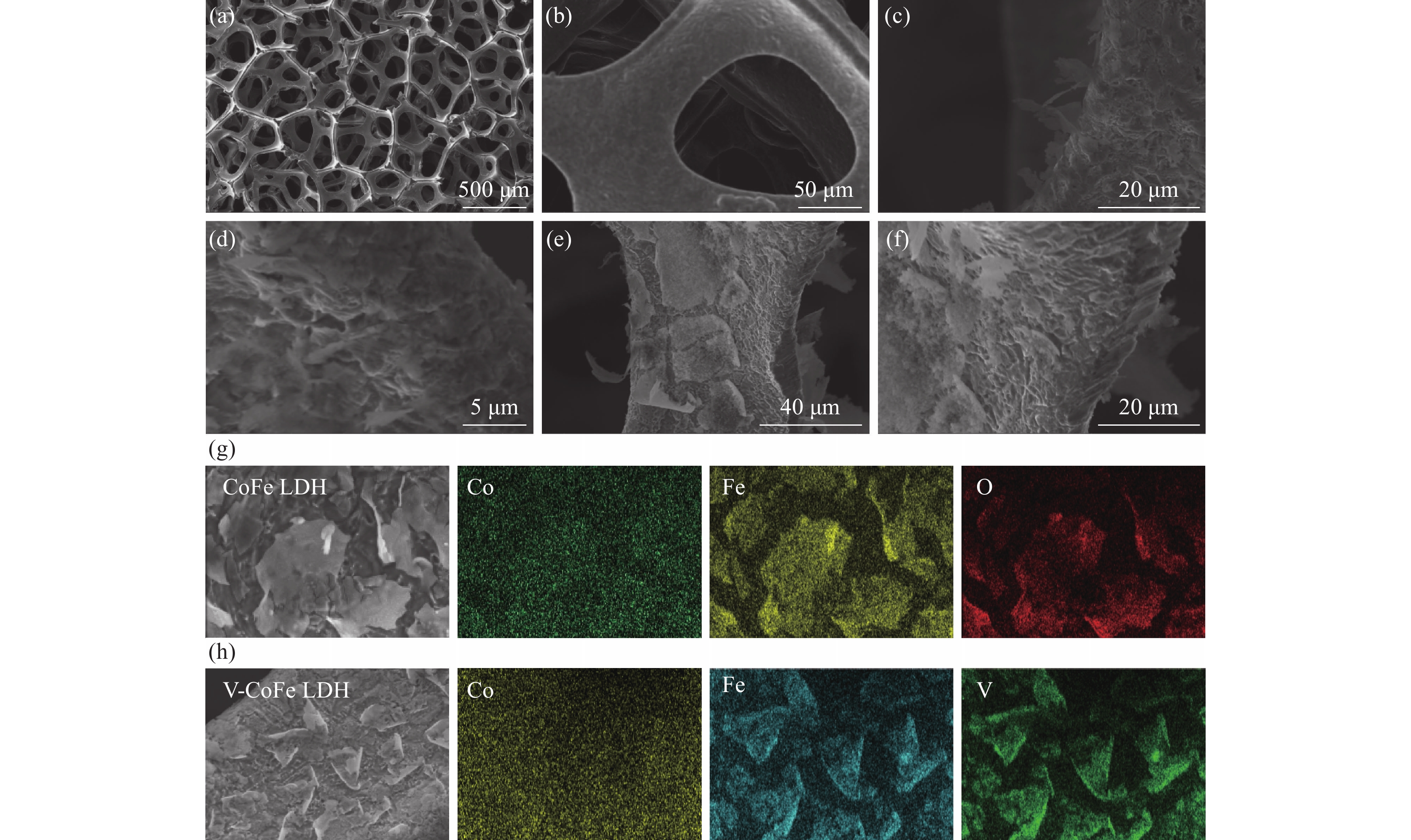
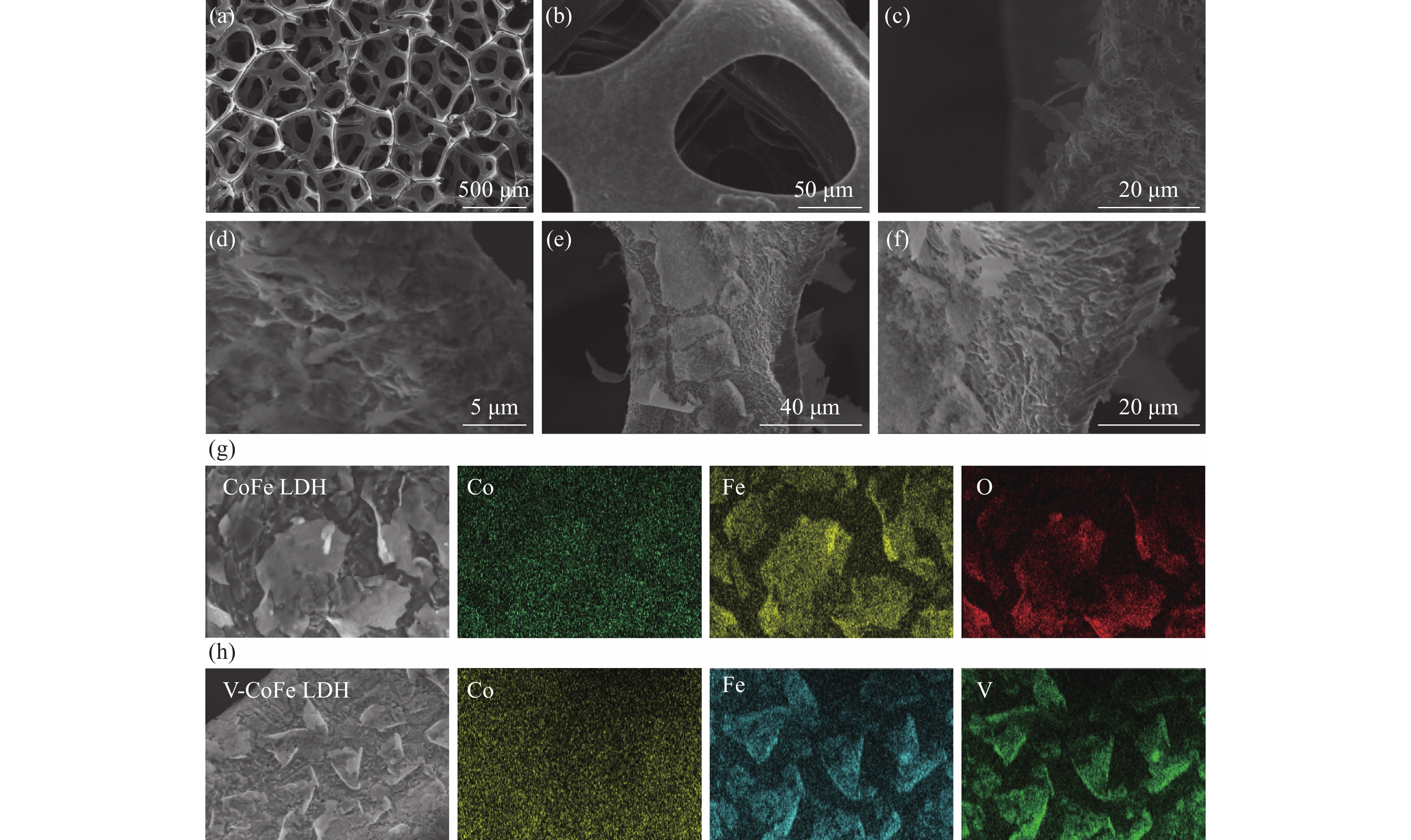
1130 、1380 cm−1附近出现的峰分别为CO32-的对称伸缩振动峰和非对称伸缩振动峰,可进一步证明该水滑石为CO32−插层;观察到在3200 cm−1和1600 cm−1左右处出现了羟基吸收峰,并且水滑石的骨架振动吸收峰在1000 ~500 cm−1范围内也有出现,上述试验结果证明成功合出了CO32−插层的CoFe LDH和V-CoFe LDH。无论是钴铁还是钒掺杂钴铁水滑石,均表现出碳酸根的特征峰,这一结果与XRD是一致的。通过SEM进一步对所制备的样品产物进行微观形貌表征,如图2所示,无论是钴铁水滑石还是钒掺杂钴铁水滑石,均表现出典型的二维片状结构,其片径大约为600 nm。根据EDS结果表明,所制备的钒掺杂钴铁水滑石中,V、Co、Fe等元素都均匀地分散在纳米片上,上述结果均说明成功地制备出了CoFe LDH和V-CoFe LDH样品。
2.2 CoFe LDH和V-CoFe LDH的OER电催化性能测试
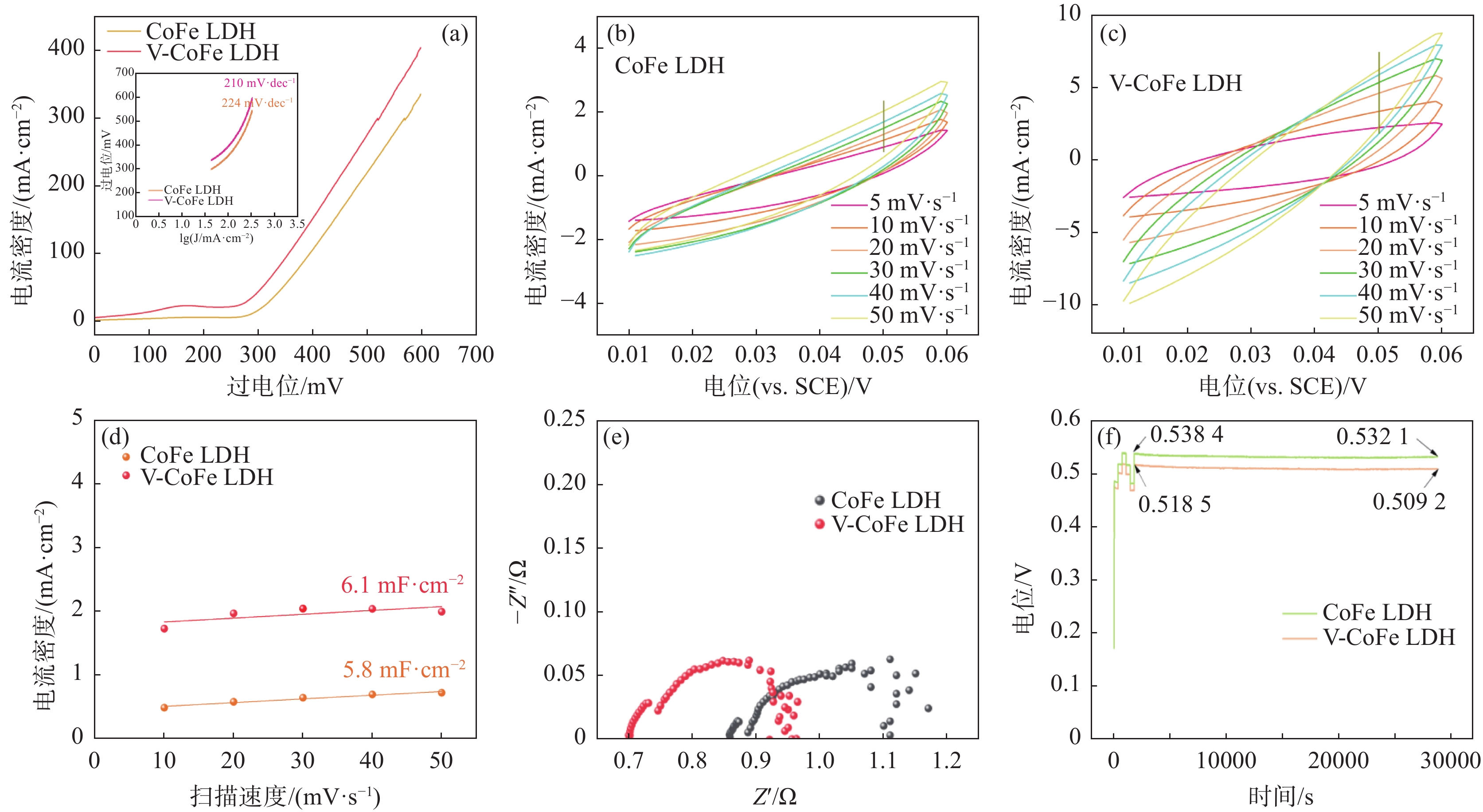
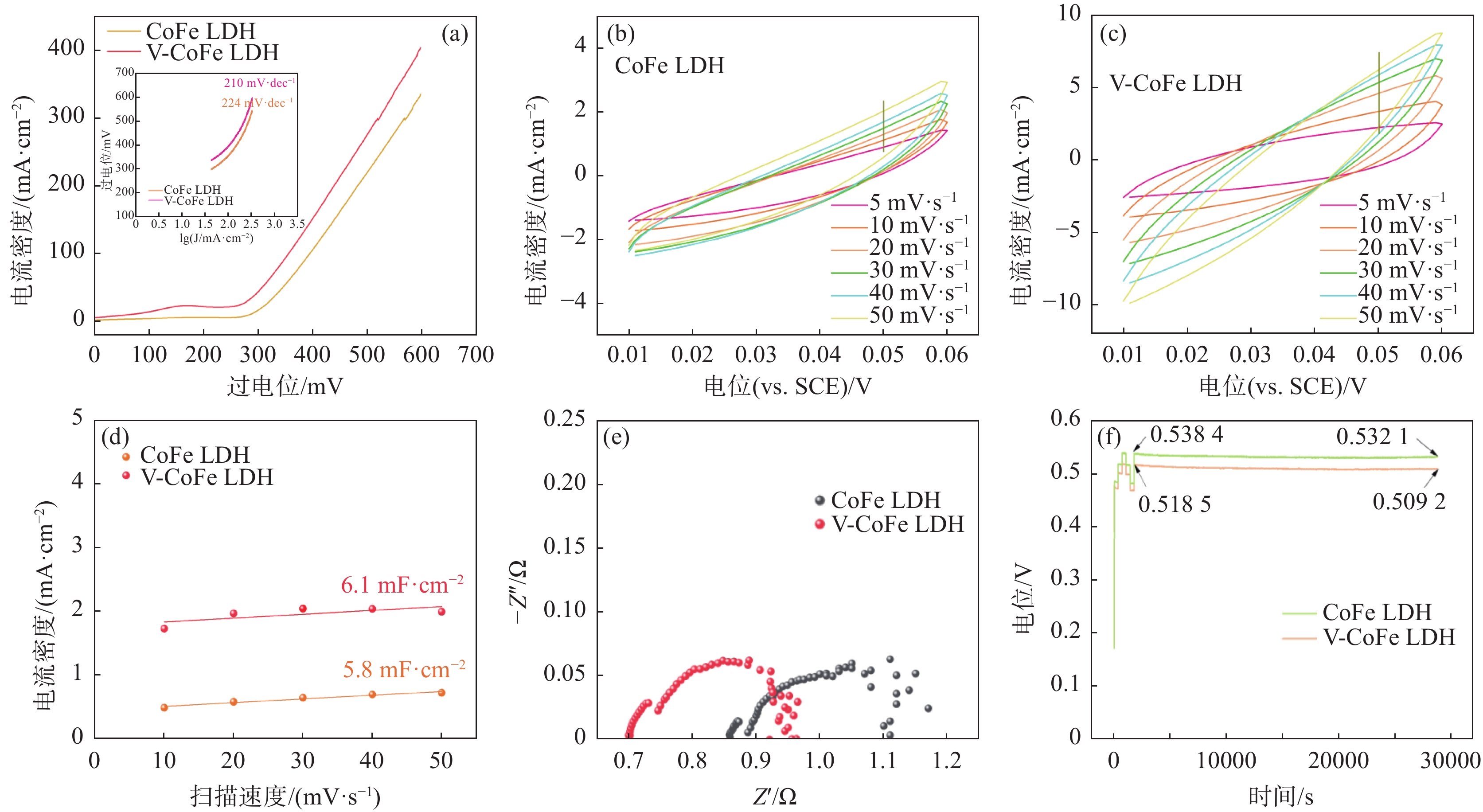
为了更好的研究和比较CoFe LDH和V-CoFe LDH 的 OER 催化性能,在室温条件下,以1.0 mol/L KOH 溶液为电解液,三电极系统条件下测试材料的OER性能。如图3(a)所示,采用扫描速率为10 mV·s−1的线性扫描伏安法得到LSV曲线,可看到在相同过电位下,V-CoFe LDH的性能要优于CoFe LDH电催化剂,当电流密度为100 mA·cm−2时,CoFe LDH和V-CoFe LDH 的过电位分别为396 mV和356 mV,这也证明了V掺杂CoFe LDH有利于降低反应的过电势,提高电解水的效率。为得到样品的 Tafel 斜率,通过图3(a)数据转化得到图3(a)内的插图,可以用来判断材料OER动力学过程的快慢,其中 V-CoFe LDH的Tafel斜率为210 mV·dec−1,CoFe LDH的Tafel斜率为224 mV·dec−1,这表明 V-CoFe LDH复合材料的 OER 反应动力学过程更快。
除反应动力学过程外,活性位点的数量也可以影响 OER 反应活性。为研究不同材料的活性比表面积,双电层电容(Cdl)被用来衡量电化学反应活性比表面积(ECSA)的大小,而 Cdl 是不同扫描速率下 CV 曲线的电流值与扫描速率的关系图计算得到的。图3(b)(c)为 CoFe LDH和V-CoFe LDH在0.01~ 0.06 V(vs. SCE)内不同扫速(5、10、20、30、40、50 mV·s−1)下的循环伏安图。用电压在0.05 V(vs. SCE)处的电流差值对不同扫速作图即可得到图3(d),其斜率高低表示材料的Cdl大小。可以看到其中 V-CoFe LDH的Cdl值(6.1 mF·cm−2)高于CoFe LDH(5.8 mF·cm−2)。这说明V-CoFe LDH的双电层电容更大,所具有的活性位点数目也最多,具有更大的电化学表面,说明V的掺杂有助于增强OER性能。
为进一步研究电催化剂的性能,EIS图(3(e))被用来衡量催化剂发生OER时电子转移速率的快慢,根据测试结果,V-CoFe LDH的EIS图中圆的半径小于CoFe LDH,这说明V-CoFe LDH电极的阻抗更小,导电性更好,掺钒后有利于提高电子转移速率。
此外电催化剂的稳定性也非常重要,是决定该催化剂能否工业化的关键条件,因此我们采用了恒电流方法来测试电催化剂的稳定性。如图3(f)所示,在电流密度为100 mA·cm−2的情况下,经过7.5 h的稳定性测试后,V-CoFe LDH电极的电位降低了1.79%,而CoFe LDH电极的电位降低了1.17%,两种电极的稳定性都较好,且V-CoFe LDH电极的电位整体比CoFe LDH电极的电压低,故其过电位更低,电化学性能更好。
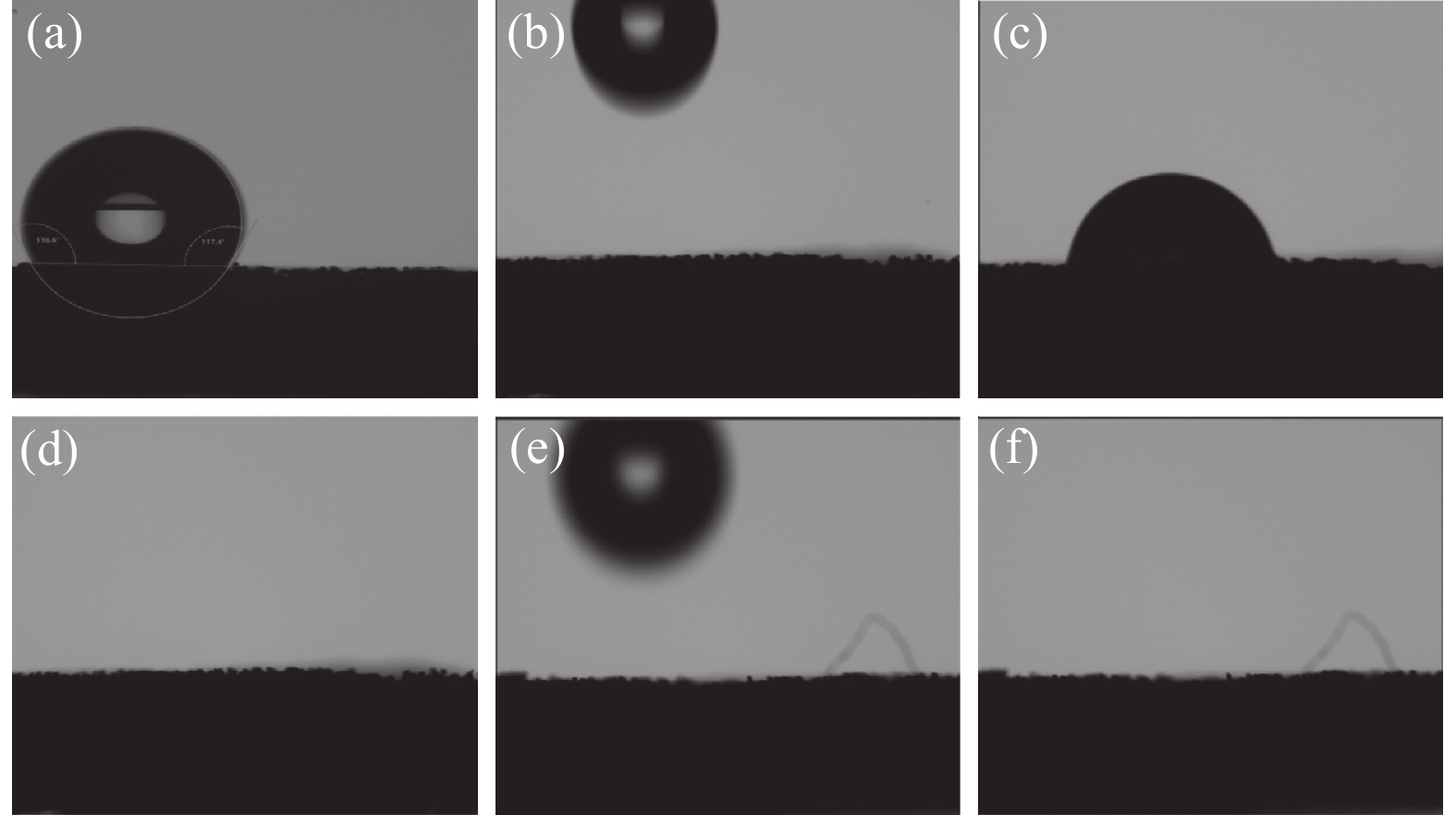
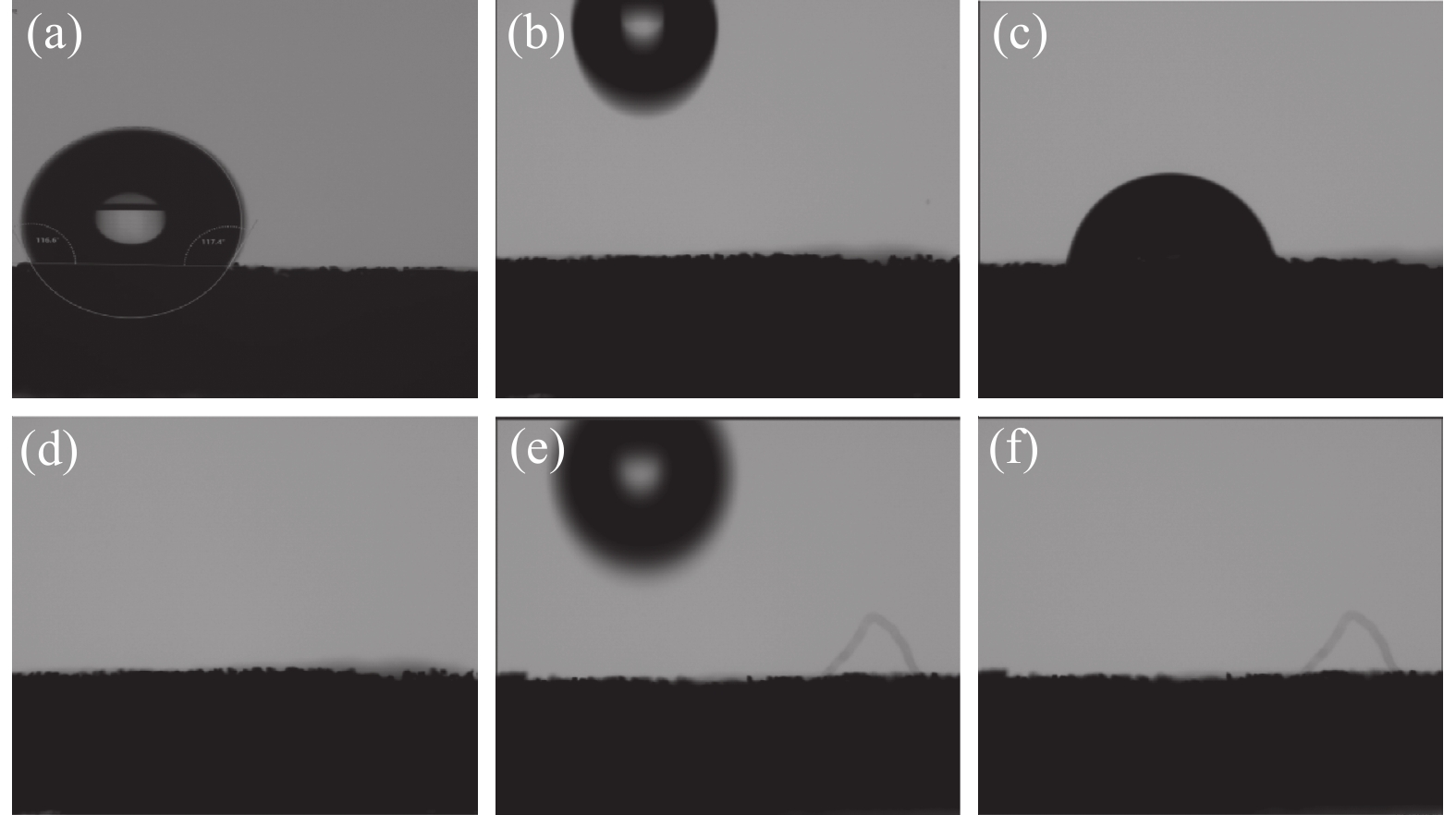
为了探究材料的亲水性变化,对经预处理过的NF、NF负载CoFe LDH和NF负载V-CoFe LDH进行了接触角测试,图4(a)为1 mol/L的KOH电解液与NF之间的接触角,图4(b)~(d)为NF负载CoFe LDH的接触角测试过程,图4(e)(f)为NF负载V-CoFe LDH的接触角测试过程,结果表明NF的亲水性最差,而后两种分别负载CoFe LDH和V-CoFeLDH两种材料都具有极佳的亲水性,观察接触角测试过程的慢放帧可以发现,V-CoFe LDH相对于CoFe LDH有更好的亲水性,电解液滴上后立马被润湿,这个过程非常快,几乎无法捕获到润湿的过程,表明V的掺杂不仅优化了材料的电子结构,同样也优化了传质过程,增强了催化剂与电解液的接触。
综上所述,将V元素引入CoFe LDH电催化剂中后,V-CoFe LDH电催化剂的析氧性能有所提升,可能的主要原因如下:①钒元素的掺杂改变了CoFe LDH的电子结构,提升了材料的电子导电性;②钒元素的掺杂使得V-CoFe LDH中有更多的电化学活性位点暴露出来;③钒元素的掺杂促进了V-CoFe LDH对电解液的吸附,有利于电催化反应的发生。
2.3 CoFe LDH和V-CoFe LDH的DFT理论计算
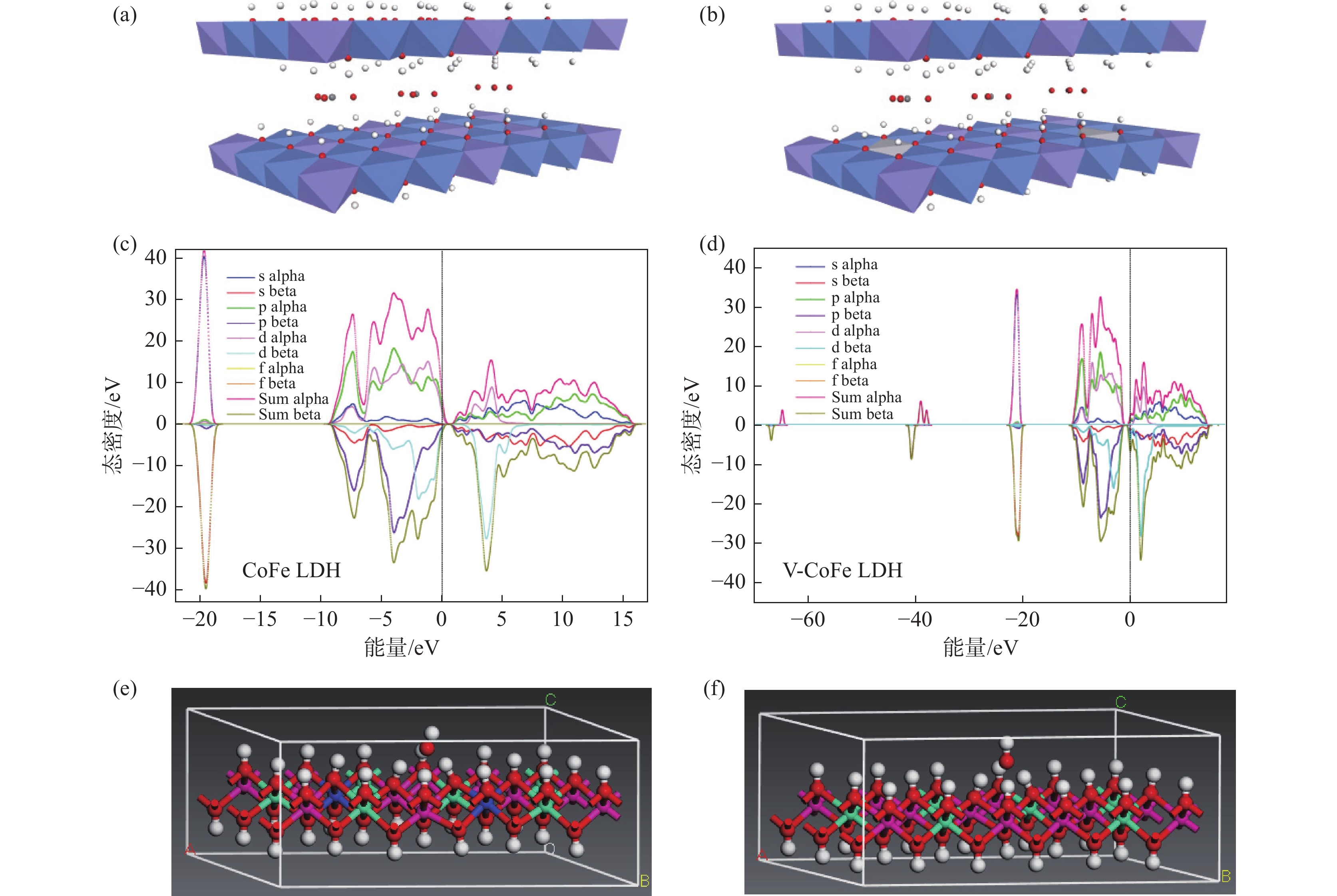
为了进一步的探究V元素掺杂对CoFe LDH电化学性能的影响,我们借助DFT理论计算进行了理论模拟分析,相关晶体结构模型如图5(a)(b)所示。通过比较V元素掺杂前后电子结构的变化,进一步解释V元素掺杂有益于CoFe LDH电化学性能的提升。相应结果如图5(c)(d)所示,通过DOS曲线分析可以看出,CoFe LDH在费米能级附近具有明显的能带间隙,表现出典型的半导体结构。同时,相较于CoFe LDH,V-CoFe LDH在费米能级附近具有更小的能带间隙,表现出更好的导电性,这一结果与EIS曲线是一致的。该能带间隙减低的原因可能归因于V元素的引入,从DOS图可以看出,V元素的d轨道在费米能级附近具有较大的电子云密度分布,减小了导带与价带之间的间隙,从而促进了导电性的提升[21]。
同时,DFT计算还可以解释V元素的引入对电解液润湿性能的影响(本文主要考虑以Fe作为主要活性位点讨论[22])。如图5(e)(f)所示分别为CoFe-LDH和V-CoFe LDH对OH−的吸附模型,根据公式(3)计算出CoFe-LDH和V-CoFe LDH对OH−的相应吸附能(表1)分别为−
4.2764 eV和−7.7834 eV。V-CoFe LDH的吸附能更低,说明V-CoFe LDH对OH−的吸附能力越强。即V元素的掺杂促进了CoFe LDH对OH−的吸附,使得材料表现出更好的电解液润湿性,这一结果与接触角测试结果也是一致的。同时,通过计算Mulliken电荷分布,进一步说明电极与电解液表面润湿性提升的原因。根据Mulliken电荷分布结果表明,在CoFe LDH 中Fe原子的电荷量为1.015 e,而在V-CoFe LDH中Fe原子电荷量为1.057 e,即V元素的引入使得Fe原子的电荷量变大,促进了材料的活性位点(Fe)与OH−之间的静电吸附作用,故而提升了其亲水性。表 1 吸附能的DFT计算结果Table 1. DFT calculation results of adsorption energyeV $E_{({\mathrm{Bulk-OH}}^-)} $ $E_{({\mathrm{Bulk}})} $ $E_{{\mathrm{OH}}^-} $ $E_{{\mathrm{ads}}} $ CoFe LDH − 35140.4510 − 34685.1275 − 451.0471 − 4.2764 V-CoFe LDH − 36853.1392 − 34685.1275 − 451.0471 − 7.7834 $$ E_{\text {ads }}=E_{\left(\text {bulk-OH}^-\right)}-E_{(\text {bulk })}-E_{\left(\mathrm{OH}^-\right)} $$ (3) 正是由于V元素的引入,使得V-CoFe LDH具有更优异的电化学比表面积、更高导电性和电解液润湿性,因此,表现出更优异的OER性能。
3. 结论
1)基于电沉积的方法,设计了一种金属掺杂方法来优化过渡金属氢氧化物材料的OER性能。V掺杂的CoFe LDH表现出优异的OER性能,在100 mV·cm−2下具有0.35 V的小过电位,更低的塔菲尔斜率,更大的电化学活性比表面积。
2)DFT研究结果表明,钒元素的掺杂优化了CoFe LDH中的电子结构,提升了材料的电子导电性,此外,电化学性能测试结果也表明钒元素的掺杂使得 V-CoFe LDH中有更多的电化学活性位点暴露出来,因此其具有更大的电化学活性比表面积,根据接触角测试和吸附能的计算结果,说明钒元素的掺杂还促进了材料对电解液的吸附,降低了吸附能,这也更有利于电催化反应的发生。
-
表 1 吸附能的DFT计算结果
Table 1. DFT calculation results of adsorption energy
eV $E_{({\mathrm{Bulk-OH}}^-)} $ $E_{({\mathrm{Bulk}})} $ $E_{{\mathrm{OH}}^-} $ $E_{{\mathrm{ads}}} $ CoFe LDH − 35140.4510 − 34685.1275 − 451.0471 − 4.2764 V-CoFe LDH − 36853.1392 − 34685.1275 − 451.0471 − 7.7834 -
[1] Wang Wei, Xu Xiaomin, Zhou Wei , et al. Recent progress in metal-organic frameworks for applications in electrocatalytic and photocatalytic water splitting[J]. Advanced Science, 2017,4(4):1600371. doi: 10.1002/advs.201600371 [2] Dincer I. Green methods for hydrogen production[J]. International Journal of Hydrogen Energy, 2012,37(2):1954-1971. doi: 10.1016/j.ijhydene.2011.03.173 [3] Lei Wanying, Zhou Tong, Pang Xin, et al. Low-dimensional MXenes as noble metal-free co-catalyst for solar-to-fuel production: Progress and prospects[J]. Journal of Materials Science & Technology, 2022,114:143-164. [4] Hu Yiming, Wang Zhaolong, Liu Wenjun, et al. A novel cobalt-iron-vanadium layered double hydroxide nanosheets arrays toward the superior water oxidation performance[J]. ACS Sustainable Chemistry & Engineering, 2019,7(19):16828-16834. [5] Reier Tobias, Mehtap Oezaslan, Peter Strasser. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: A comparative study of nanoparticles and bulk materials[J]. ACS Catalysis, 2012,2:1765-1772. doi: 10.1021/cs3003098 [6] Youngmin Lee, Jin Suntivich, Kevin J May, et al. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions[J]. Journal of Physical Chemistry Letters, 2012,3:399-404. doi: 10.1021/jz2016507 [7] Antolini Ermete. Iridium as catalyst and cocatalyst for oxygen evolution/reduction in acidic polymer electrolyte membrane electrolyzers and fuel cells[J]. ACS Catalysis, 2014,4(5):1426-1440. doi: 10.1021/cs4011875 [8] Kötz R, Lewerenz H J, Stucki S, et al. XPS studies of oxygen evolution on Ru and RuO2 anodes[J]. Journal of the Electrochemical Society, 1983,130:825-829. doi: 10.1149/1.2119829 [9] Wang Shenggao, Wang Tao, Wang Xujie, et al. Intercalation and elimination of carbonate ions of NiCo layered double hydroxide for enhanced oxygen evolution catalysis[J]. International Journal of Hydrogen Energy, 2020,23(45):12629-12640. [10] Isabela C Man, Su Haiyan, Federico Calle Vallejo, et al. Universality in oxygen evolution electrocatalysis on oxide surfaces[J]. Chem Cat Chem, 2011, 3(7): 1159-1165. [11] Long Xia, Li Jinkai, Xiao Shuang, et al. A strongly coupled graphene and FeNi double hydroxide hybrid as an excellent electrocatalyst for the oxygen evolution reaction[J]. Angewandte Chemie(International Ed), 2014, 53(29): 7584-7588. [12] Long Xia, Xiao Shuang, Wang Zilong, et al. Co intake mediated formation of ultrathin nanosheet of transition metal LDH-an advanced electrocatalysts for oxygen evolution reaction[J]. Chem Commun, 2015,1(6):1120-1123. [13] Ding Yangyang, Du Xiaoqiang, Zhang Xiaoshuang, et al. Controllable synthesis of CoFeMo layered double hydroxide nanoarrays for promoting oxygen evolution reaction[J]. Dalton Transactions, 2020,49:15417-15424. doi: 10.1039/D0DT03182H [14] Gong Ming, Li Yanguang, Wang Hailiang, et al. An advanced NieFe layered double hydroxide electrocatalyst for water oxidation[J]. Journal of the American Chemical Society, 2013,135(23):8452-8455. doi: 10.1021/ja4027715 [15] Yu Xiaowen, Zhang Miao, Yuan Wenjing, et al. High-performance three-dimensional Ni-Fe layered double hydroxide/graphene electrode for water oxidation[J]. Journal of Materials Chemistry A, 2015,3(13):6921-6928. doi: 10.1039/C5TA01034A [16] Li Kaiyue, Guo Dong, Kang Jianyu, et al. Hierarchical hollow spheres assembled with ultrathin CoMn double hydroxide nanosheets as trifunctional electrocatalyst for overall water splitting and Zn air battery[J]. ACS Sustainable Chemistry & Engineering, 2018,6(11):14641-14651. [17] Yang Yang, Dang Lianna, Shearer Melinda J, et al. Highly active trimetallic NiFeCr layered double hydroxide electrocatalysts for oxygen evolution reaction[J]. Advanced Energy Materials, 2018,8(15):1703189. doi: 10.1002/aenm.201703189 [18] Feng Yihan, Li Zichuang, Li Shanlin, et al. One stone two birds: Vanadium doping as dual roles in self-reduced Pt clus-ters and accelerated water splitting[J]. Journal of Energy Chemistry, 2022,66:493-501. doi: 10.1016/j.jechem.2021.08.061 [19] Wang Shenggao, Wang Tao, Wang Xujie, et al. Intercalation and elimination of carbonate ions of NiCo layered double hydroxide for enhanced oxygen evolution catalysis[J]. International Journal of Hydrogen Energy, 2020,45(23):12629-12640. doi: 10.1016/j.ijhydene.2020.02.212 [20] Wang Bo, Gareth R Williams, Chang Zheng, et al. Hierarchical NiAl layered double hydroxide/multiwalled carbon nanotube/nickel foam electrodes with excellent pseudocapacitive properties[J]. ACS Applied Materials & Interfaces, 2014,6:16304-16311. [21] Wang Yuhang, Chen Long, Yu Xiaomin, et al. Superb alkaline hydrogen evolution and simultaneous electricity generation by Pt-decorated Ni3N nanosheets[J]. Advanced Energy Materials, 2016,7:1601390. [22] Tian Yang, Bi Yongming, Qin Bangchang, et al. Density functional theory investigation of oxygen evolution reaction on the NiFe-LDHs (100) surface[J]. Joural of Advances in Physical Chemistry, 2017,6(2):75-83. (田阳, 毕永民, 秦邦昌, 等. NiFe-LDHs催化氧气析出反应的密度泛函理论研究[J]. 物理化学进展, 2017,6(2):75-83. doi: 10.12677/JAPC.2017.62010Tian Yang, Bi Yongming, Qin Bangchang, et al. Density functional theory investigation of oxygen evolution reaction on the NiFe-LDHs (100) surface[J]. Joural of Advances in Physical Chemistry, 2017, 6(2): 75-83. doi: 10.12677/JAPC.2017.62010 -






 下载:
下载:




 下载:
下载: