Research progress of targeted extraction of vanadium by functional ionic liquids
-
摘要: 溶剂萃取法是提钒最常用的分离富集方法。传统的工业萃钒方法存在萃取效果不理想、选择性差以及环境和安全方面等问题。离子液体具有宽液程、低蒸汽压不易挥发、物化性质可调、易于功能性设计、电化学窗口宽、导电性好、热稳定性高等诸多优点,可实现钒的靶向高效萃取。综述了离子液体萃取回收钒的研究进展,重点关注了咪唑类离子液体和季铵盐类离子液体的物化性质、合成方法与萃钒机理。钒的萃取机理主要包括阴离子交换和中性络合等机制,通过不同离子液体的特性,钒的萃取效率和选择性得以优化。通过对离子液体在提钒过程中的机理分析和应用案例的揭示,以期为钒提取领域的研究和应用提供参考。同时,也指出了离子液体萃取剂在成本、大规模应用等方面面临的一些挑战,需要进一步的研究和发展。Abstract: The solvent extraction method is the most commonly used technique for the separation and enrichment of vanadium. However, traditional industrial vanadium extraction methods encounter issues such as unsatisfactory extraction efficiency, poor selectivity, and environmental and safety concerns. Ionic liquids possess many advantages, including a wide liquid range, low vapor pressure, non-volatility, tunable physical and chemical properties, ease of functional design, a wide electrochemical window, good conductivity, and high thermal stability, enabling targeted and efficient extraction of vanadium. This review summarizes the research progress on the extraction and recovery of vanadium using ionic liquids, with a focus on the physical and chemical properties, synthesis methods, and extraction mechanisms of imidazolium and quaternary ammonium ionic liquids. The extraction mechanisms of vanadium mainly include anion exchange and neutral complexation, which optimize the extraction efficiency and selectivity of vanadium through the unique properties of different ionic liquids. By analyzing the mechanisms of ionic liquids in the vanadium extraction process and revealing application cases, this review aims to provide references for research and applications in the field of vanadium extraction. Additionally, it highlights the challenges faced by ionic liquid extractants in terms of cost and large-scale application, indicating that further research and development are needed.
-
0. 引言
钒是一种可服务于战略性新兴产业技术的关键金属,在冶金、化工、新能源、航空航天等领域得到了广泛应用。随着科技和经济的不断发展,对钒(尤其是高纯度钒)的需求将进一步增加[1−3]。目前,全湿法直接酸浸提取钒技术已经成为主流趋势[4−6],该技术省去了高温焙烧的步骤,更环保低碳,且具有较高的浸出率。然而,如果不进行焙烧直接酸浸,通常需要高浓度酸浸出,这会导致酸浸过程缺乏选择性,直接酸浸液中的杂质较多,难以净化提纯,严重影响最终高纯度钒产品的质量,从而限制了高纯度钒的生产[7−10]。
净化提纯含钒溶液通常采用化学沉淀法、离子交换法和溶剂萃取法[11]。化学沉淀法要求溶液性质高、难以控制除杂剂量[12];离子交换法设备昂贵、“中毒”现象严重,限制了广泛应用[13];而溶剂萃取法因富集程度高、除杂彻底且操作方便,被广泛应用,并具有很好的工业化前景[14]。传统的有机萃取剂存在环境污染、有毒、火灾隐患等问题,且性能固定、针对性差,难以适应复杂溶液,而且在高酸条件下分离效率低,严重影响产品纯度。为了解决这些问题,离子液体作为一种新型绿色萃取剂备受研究关注。其具有诸多优点,如宽液程、低挥发性、性质可调、易于设计、导电性好、热稳定性高,较传统有机溶剂具有显著优势,在萃取领域应用前景良好[15−17]。功能性离子液体对钒离子表现出更高的萃取力和更优越的靶向性,尤其是咪唑类和季铵盐类离子液体备受关注。
鉴于此,笔者对离子液体的类型、物化性质和合成方法进行了概述,重点介绍了功能性离子液体咪唑类和季铵盐类在萃钒方面的基本原理和研究进展。通过对离子液体在提钒过程中的机理分析和应用案例的揭示,以期为钒提取领域的研究和应用提供参考,为实现高效、环保的钒回收提供新的思路和方法。
1. 功能性离子液体的类型及物化性质
离子液体(ILs),作为一类独特的有机盐化合物,由特定的阳离子与阴离子构成,其显著特征在于熔点远低于常规盐类的100 ℃界限。这类液体的流动性得益于其阴阳离子间显著的体积不匹配及相对减弱的静电相互作用力。ILs凭借其卓越的物理化学性质,如低易燃性、出色的热稳定性及极低的挥发性,成为传统挥发性有机溶剂的理想替代品,展现出显著的环保优势[18−19]。在萃取科学领域,ILs的应用尤为广泛,不仅能够作为高效的萃取剂直接参与目标物质的分离,还能作为稀释剂调节体系浓度,甚至作为协萃剂增强萃取效率[20−21]。具体而言,根据阳离子的化学结构差异,常用的萃取型ILs可细分为四大类:季膦盐系列、季铵盐系列、咪唑系列以及吡啶系列。在萃取钒的过程中,常用的离子液体见表1,其中咪唑类和季铵盐类离子液体表现出对钒的高萃取率和高选择性而受到广泛关注。
表 1 常用于萃钒的离子液体类型Table 1. Types of ionic liquids commonly used in vanadium extraction离子液体类型 离子液体类别名称及相关化学式 阳离子 咪唑 
苯并咪唑盐 
铵 
吡啶盐 
阴离子 六氟磷酸根( Ⅴ ) 
四氟硼酸根 
卤素阴离子 
醋酸盐 
硝酸盐 
2,2,2 -三氟乙酸 
双离子 咪唑类
([C6mim][PF6])
([C8mim][PF6])C12H23ClN2
([Omim]Cl)
C12H23BrN2
([Omim]Br)
C12H23BF4N2
([Omim][BF4])
([Bmim]PF6)
Bmim Cl-AlCl3季铵盐类 
C25H54ClN
[A336][P204]
[A336][P507]离子液体凭借其独树一帜的物理化学特性在诸多领域展现出广泛的应用潜力,相关文献记载丰富[22−28]。此类溶剂展现出如低挥发、高热稳定、蒸汽压低、电化学窗口宽广、极性可调以及液相区域广泛的诸多优势特性。鉴于阳离子与阴离子间的灵活互换与定制化能力,理论上可构建出约1018种不同的离子液体组合,从而赋予了其“设计溶剂”的美誉[29]。在验证理论模型或筛选适宜的离子液体萃取剂时,其物理与化学属性显得尤为重要。具体而言,熔点、体积密度、分子量等核心参数直接关联于萃取过程中的关键环节,如溶剂的甄选、萃取效率的优化以及操作条件的设定等。针对提钒工艺,常用的咪唑类及季铵盐类功能性离子液体的详尽物化性质[30−31]已汇总于表2中,以供深入研究与参考。
表 2 常见咪唑类和季铵盐类离子液体物理化学性质Table 2. Physicochemical properties of imidazolium and quaternary ammonium ionic liquids离子液体类型 名称 熔点/ ℃ 体积密度
(20 ℃)/(g·cm−3)分子量 咪唑类 [C6mim][PF6] −73.5 1.3045 312.24 [C8mim][PF6] −70 1.2345 340.29 [Omim]Cl 12 1.01 230.78 [Omim]Br −61.9 1.17 275.23 [Omim][BF4] −80 1.09 282.13 [bmim]PF6 6.5 1.38 284.18 季铵盐类 C25H54ClN −20 0.884 404.16 2. 功能性离子液体的合成
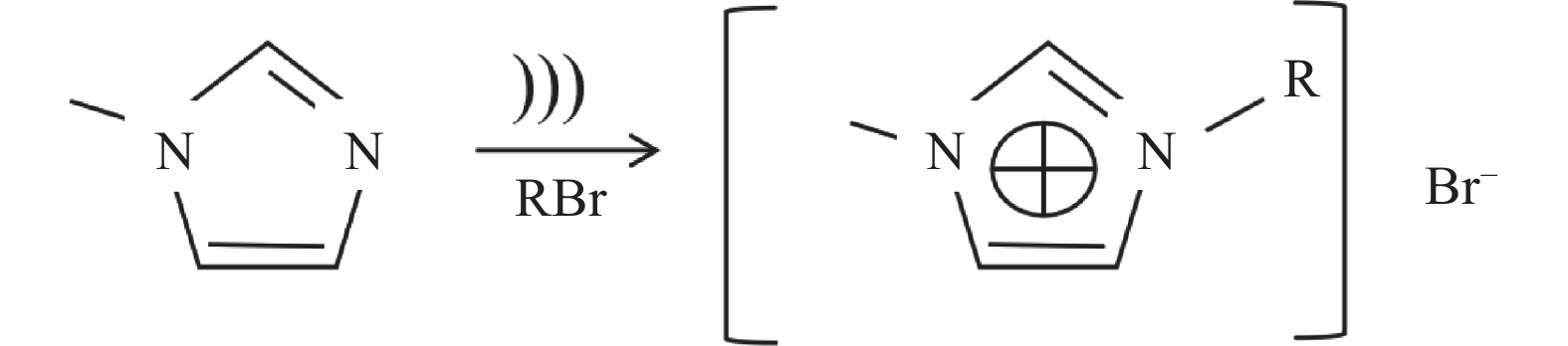
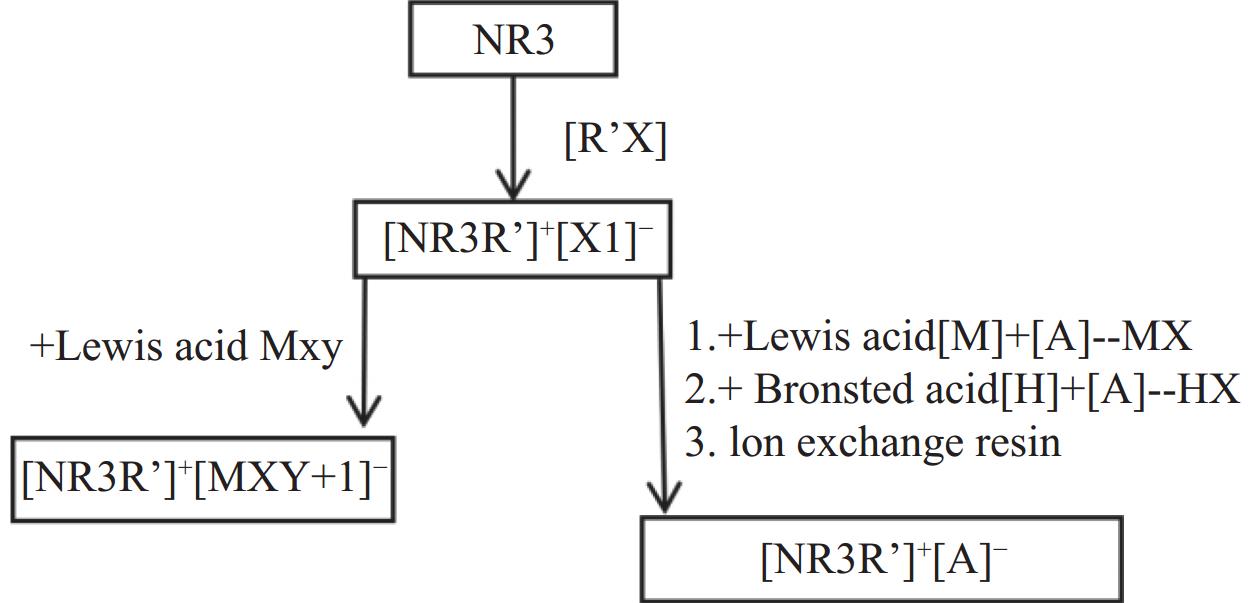
合成功能性离子液体有两种基本方法:酸碱中和法和复分解反应法[32−33]。其简化合成流程如图1所示。
 图 1 合成离子液体的方法流程[33]Figure 1. Flow chart for the synthesis of ionic liquids
图 1 合成离子液体的方法流程[33]Figure 1. Flow chart for the synthesis of ionic liquids2.1 咪唑类功能性离子液体的合成方法
2.1.1 [C6mim][PF6]和[C8mim][PF6]
[C6mim][PF6]和[C8mim][PF6]的合成方法已经被充分验证[34−37]。首先需要合成无色透明的中间体[C6mim][Cl]和[C8mim][Cl],其反应方程式为式(1)(2),其次在水中合成[C6mim][PF6]和[C8mim][PF6],洗涤后,旋转蒸发除掉微量水和有机杂质,得到离子液体[C6mim][PF6]和[C8mim][PF6],反应式为式(3)(4)[38]。


$$ \mathrm{NaPF}_6 + \left[\mathrm{C}_6 \mathrm{mim}\right][\mathrm{Cl}] \rightarrow \mathrm{NaCl} + \left[\mathrm{C}_6 \mathrm{mim}\right]\left[\mathrm{PF}_6\right] \downarrow $$ (3) $$ \mathrm{NaPF}_6+\left[\mathrm{C}_8 \mathrm{mim}\right][\mathrm{Cl}] \rightarrow \mathrm{NaCl}+\left[\mathrm{C}_8 \mathrm{mim}\right]\left[\mathrm{PF}_6\right] \downarrow $$ (4) 2.1.2 [Omim][X] (X=Cl−、Br−、BF4−)、[Bmim]PF6
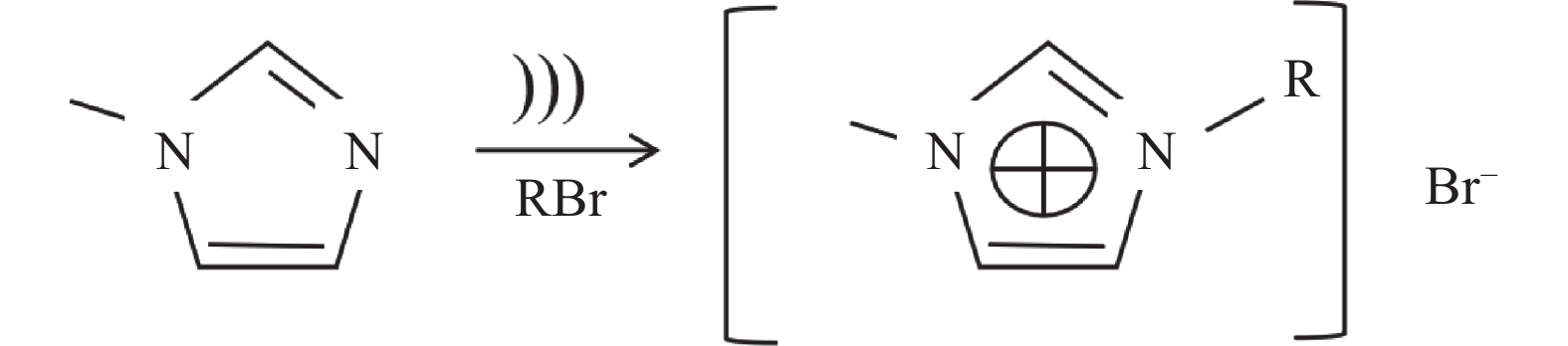
[Omim]Cl的合成步骤与[Omim]Br相同,以[Omim]Br的反应为例,反应流程如图2所示[39−41]。
 图 2 [Omim]Br的反应流程[41]Figure 2. The reaction flow chart of [Omim]Br
图 2 [Omim]Br的反应流程[41]Figure 2. The reaction flow chart of [Omim]Br[Omim][BF4]、[Bmim][PF6]的合成均属于两步合成法[42−43]。以[Bmim][PF6]为例,首先要合成中间产物[Bmim]Br,见方程式(5)。
$$ \mathrm{C}_4 \mathrm{H}_9 \mathrm{Br}+\mathrm{C}_4 \mathrm{H}_6 \mathrm{N}_2 \rightarrow \mathrm{C}_8 \mathrm{H}_{14} \mathrm{BrN}_2 $$ (5) 接下来,将[Bmim]Br与六氟磷酸钾进行混合,其反应过程遵循方程式(6)所描述的路径。在此过程中,引入了去离子水作为反应溶剂,并通入氮气以有效排除体系中的氧气。待反应彻底完成后,首先移除上层的水相部分,随后对剩余的下层有机相进行多次去离子水洗涤处理。为了确认溴离子的完全去除,采用浓度为0.05 mol/L的硝酸银(AgNO3)溶液对洗涤后的液体进行持续检测,直至溴离子不再显现。完成洗涤步骤后,对下层液体实施减压蒸馏操作,以去除残留的微量水分。随后,将处理过的液体转移至设定温度为70 ℃的恒温干燥箱中,进行长达36 h的干燥过程。通过上述一系列精细操作,最终成功制备出[Bmim]PF6产物[44−47],如式(6)。
$$ \mathrm{C}_8 \mathrm{H}_{14} \mathrm{BrN}_2+\mathrm{KPF}_6 \rightarrow[\mathrm{Bmim}] \mathrm{PF}_6\left(\mathrm{C}_8 \mathrm{H}_{14} \mathrm{F}_6 \mathrm{N}_2 \mathrm{P}\right)+\mathrm{KBr} $$ (6) 2.2 季铵盐类功能性离子液体的合成方法
2.2.1 甲基三辛基氯化铵
甲基三辛基氯化铵,又称TOMAC或A336,可以通过将甲基氯化铵和辛醇加入有机溶剂中,然后加入三乙胺作为催化剂进行合成。反应完成后,将产物分离出来并进行纯化和结晶处理,反应方程为式(7)。
$$ \begin{split} &\mathrm{CH}_3 \mathrm{ClNH}_2+3 \mathrm{C}_8 \mathrm{H}_{17} \mathrm{OH}+3 \mathrm{C}_6 \mathrm{H}_{15} \mathrm{N} \rightarrow \\ & \left(\mathrm{C}_8 \mathrm{H}_{17}\right)_3 \mathrm{CH}_3 \mathrm{NCl}+3 \mathrm{C}_6 \mathrm{H}_{15} \mathrm{N}+3 \mathrm{H}_2 \mathrm{O} \end{split} $$ (7) 2.2.2 [A336][P507]和[A336][P204]
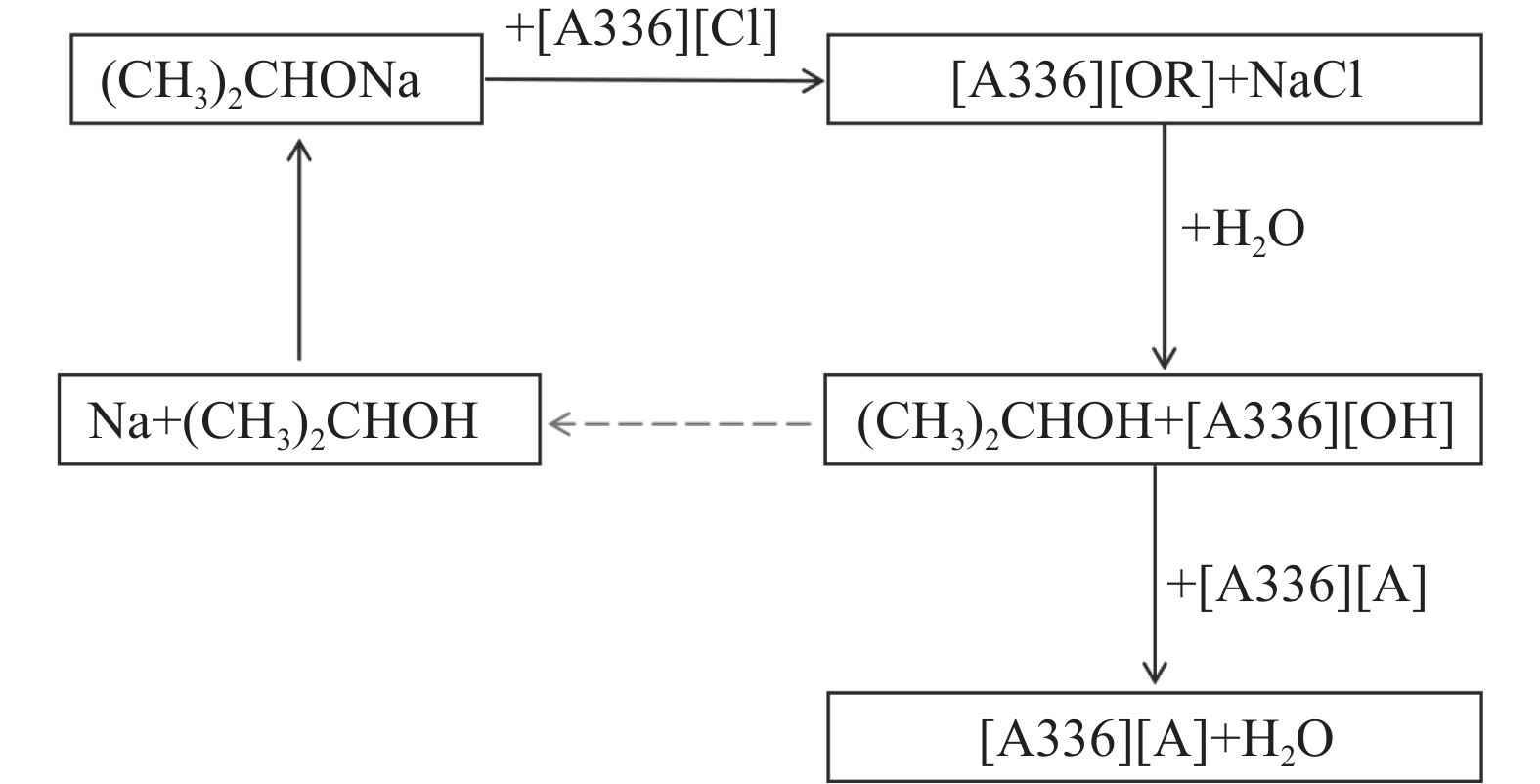
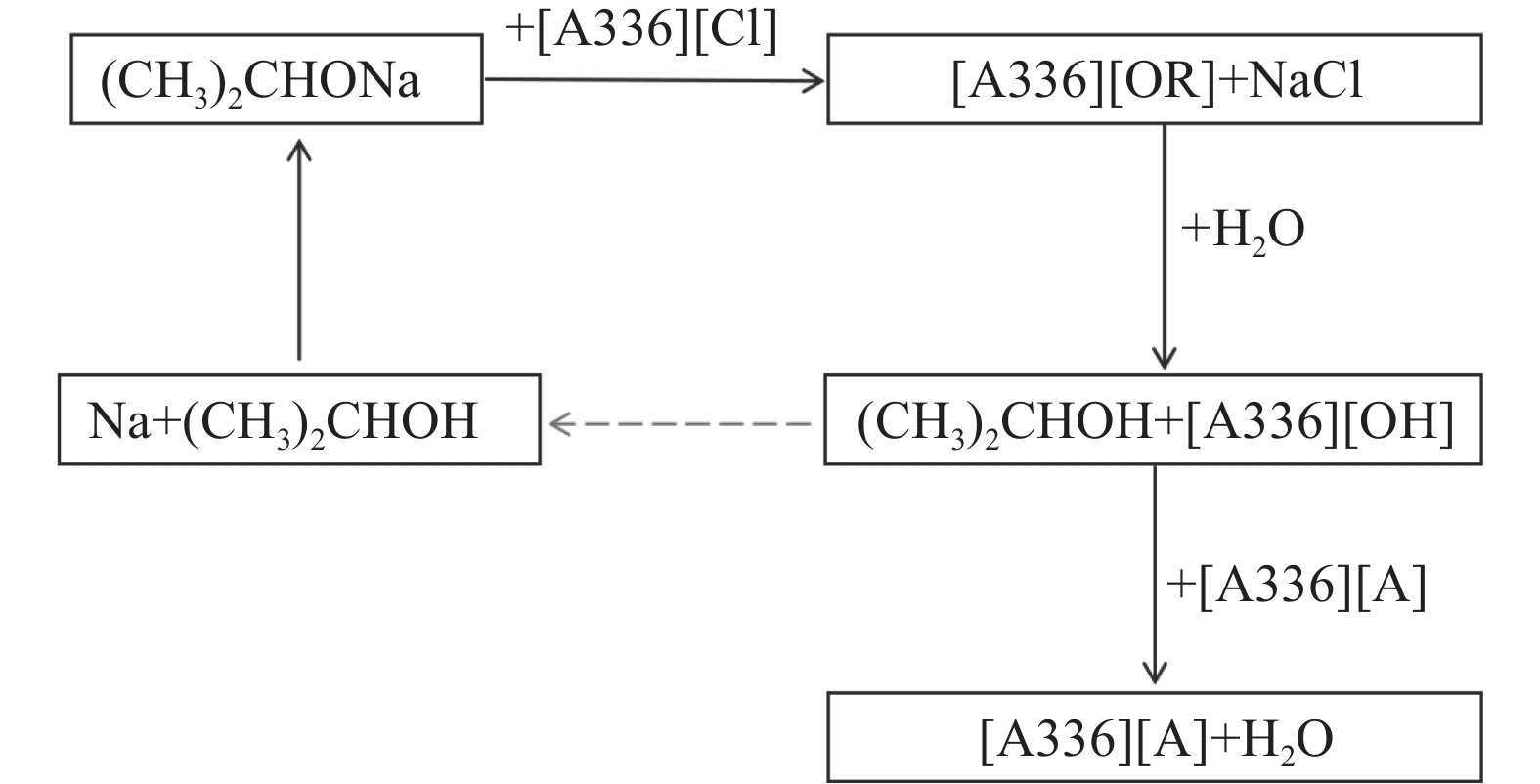
双功能基离子液体[A336][P507]和[A336][P204]可通过酸碱中和法合成,其合成流程见图3。另外,还有一种合成季铵盐双功能基离子液体的方法。该方法选用甲醇作稀释剂,用碳酸氢钠溶液作去质子剂,在80 ℃下旋转蒸发去除甲醇和水分,即可得到目标双功能基离子液体及氯化钠。过滤去除氯化钠,真空干燥即可得到目标的双功能基离子液体[48]。
3. 功能性离子液体靶向萃钒的基本原理
3.1 含钒酸浸液中的离子分析
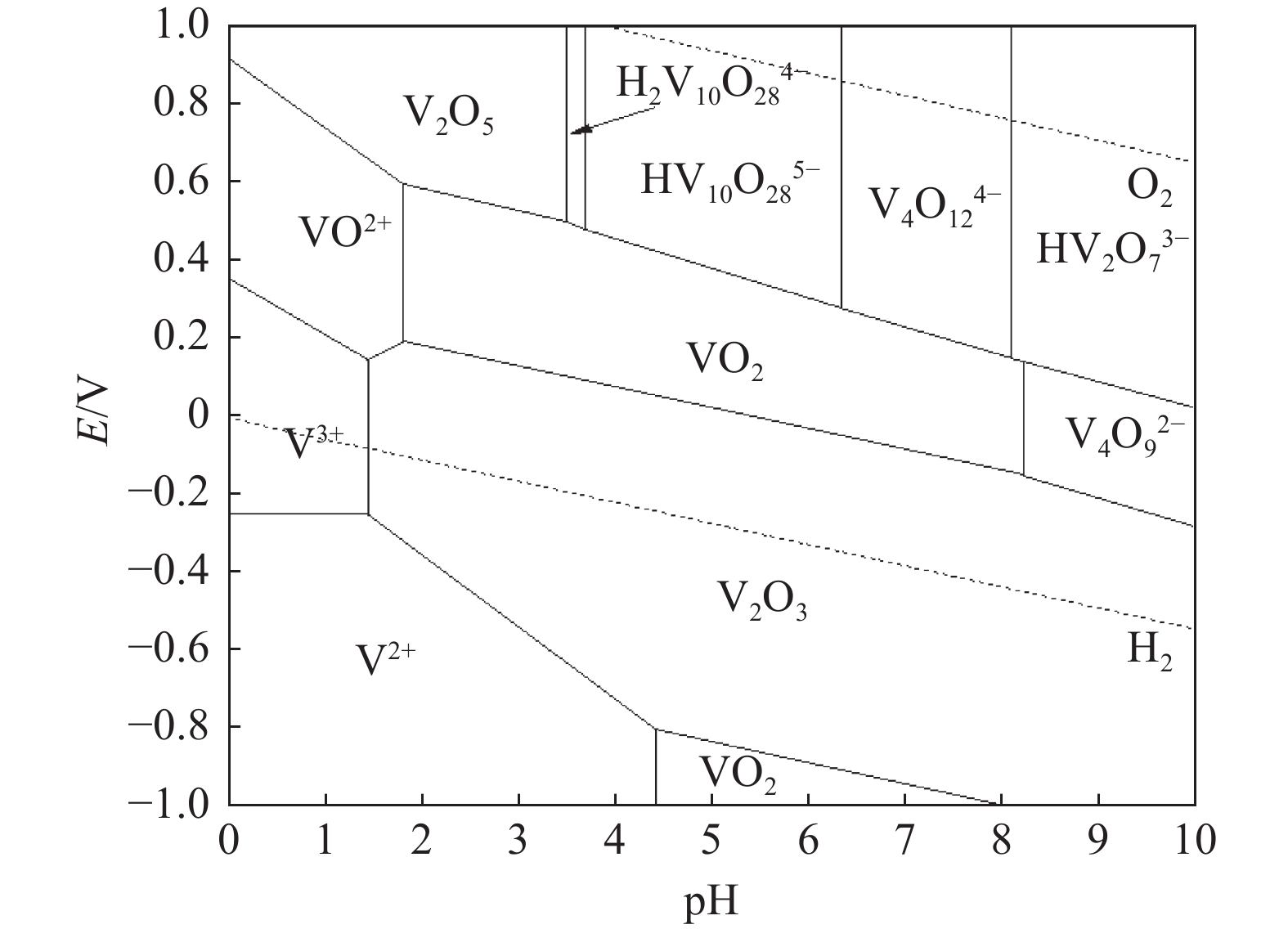
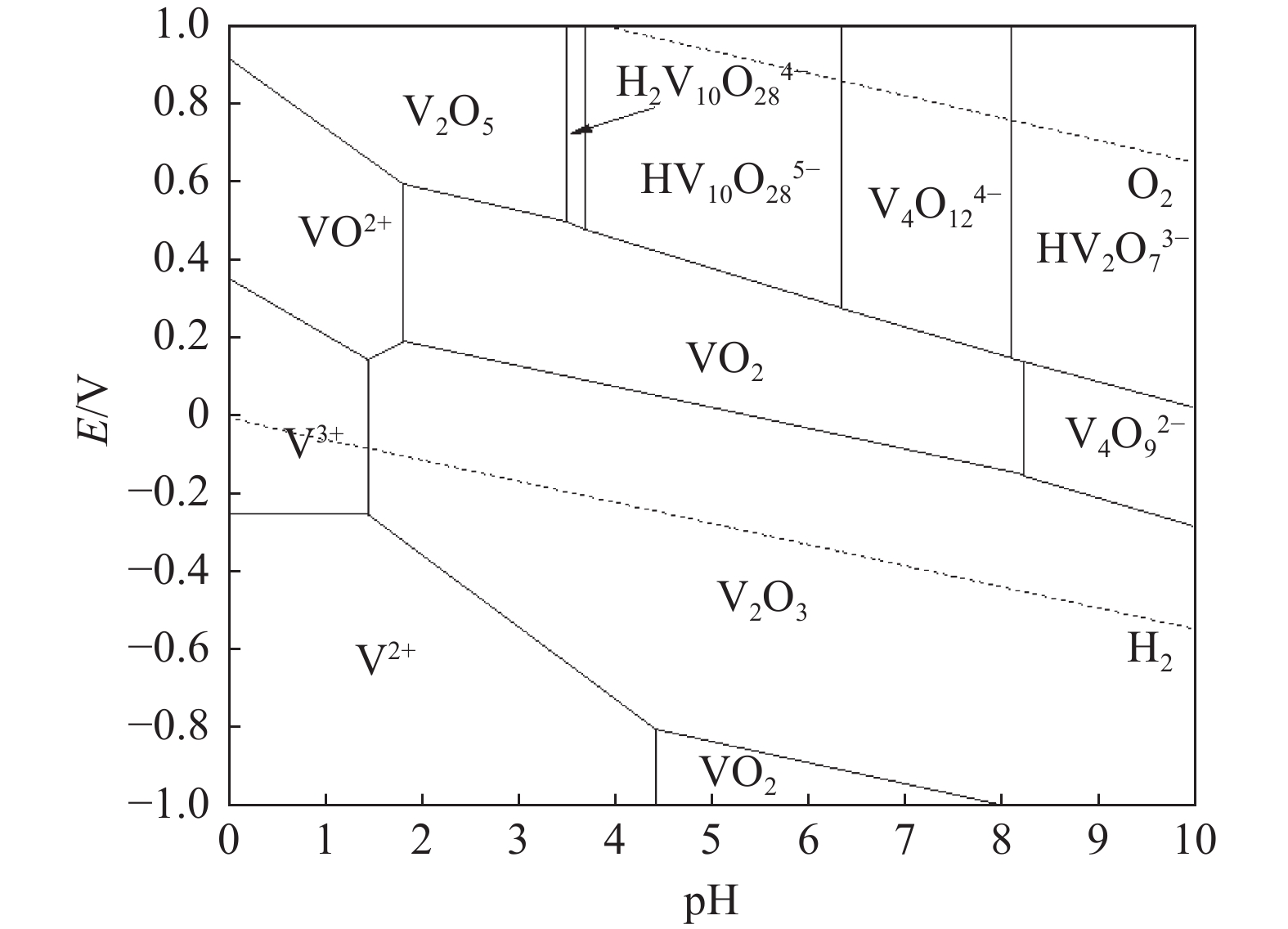
在含钒溶液中,钒主要以V(Ⅳ)和V(Ⅴ)两种价态存在,它们的存在形态与溶液pH、杂质元素等多种因素有关。Hu Yibo等[49]绘制了V-H2O在室温及1 mol/L钒浓度下的E-pH图,如图4所示。
依据图4,强酸性环境中,V(Ⅳ)元素主要以VO2+阳离子形态存在,其浓度随溶液pH值的升高而逐渐降低。具体而言,当pH值由2逐步提升至9的过程中,VO2+趋于析出。相比之下,V(Ⅴ)的存在形态更为繁复,于强碱性条件下,它主要呈现为VO43−阴离子。随着溶液酸度增强,即pH值下降,VO43−会与H+结合,依次转化为V2O74−。在pH值从12递减至9的区间内,钒主要以V2O74−、HVO42−及HV2O73−等形态共存。进一步地,当pH值从7降至4时,V4O124−与H+结合,生成具有高度聚合性和复杂三维环状结构的V10O286−离子,水溶液中呈现出鲜明的橘黄色。值得注意的是,若溶液中钒的浓度达到较高水平,钒离子将倾向于聚合成焦钒酸根离子[50]。而当pH值急剧下降至1时,钒的聚合程度显著增强,多钒酸根离子结构变得不稳定,易于被破坏,随后析出为水合五氧化二钒沉淀。最终,在极端酸性条件(pH<1)下,钒以VO2+的形式稳定存在。
3.2 咪唑类功能性离子液体萃钒机理
3.2.1 [C8mim][PF6]、[C6mim][PF6]萃钒机理
对含钒酸浸液选择合适的萃取剂,需要对萃取过程的机理进行深入研究。常见用于萃取钒的咪唑类离子液体有[C6mim][PF6]和[C8mim][PF6]等。对于[Cnmim][PF6]( n=4,6,8),其对钒的萃取能力顺序为[C8mim][PF6] > [C6mim][PF6] >[C4mim][PF6],这应该与咪唑环上烷基基团的长度有关。咪唑阳离子中n-烷基链长的增加导致ILs的疏水性增加。因此,[C8mim] [PF6]因长链的辛基而具有更强的疏水性,这有助于钒的更高分配比例。[C8mim][PF6]对V(Ⅴ)的萃取机理可能是主要物种HV10O273−和PF6−之间的阴离子交换。其反应方程见式(8)[51]。
$$ {\begin{split} & 3\left[\mathrm{C}_8 \mathrm{mim}\right] \left[\mathrm{PF}_6\right] + \mathrm{HV}_{10} \mathrm{O}_{27}{ }^{3-} = \left(\mathrm{C}_8 \mathrm{mim}\right)_3 \mathrm{HV}_{10} \mathrm{O}_{27}+\\ & 3 \mathrm{PF}_6^- \end{split} } $$ (8) 3.2.2 [Omim]Cl、[Omim]Br和[Omim][BF4]萃钒机理
研究发现[52],离子液体[Omim]Cl、[Omim]Br和[Omim][BF4]在萃取钒离子(V)时表现出不同的提取效率。随着阴离子体积的增加,使用离子液体(ILs)提取V(Ⅴ)的速率逐渐减小。这是因为较大的阴离子能容纳更多的阳离子,增强了提取过程中的复合物形成能力。同时,随着离子液体的晶格能增加,钒离子(Ⅴ)的提取速率也随之增加。阴离子的体积顺序为Cl− < Br− < BF4− < HVO42−,因此,HVO42−与Cl−、Br−和BF4−相比具有更大的体积,其与[Omim]+的络合能力也更强,可以更有效地与这些阴离子进行离子交换。此外,由于Cl−体积最小,它更倾向于与HVO42−进行离子交换,因此在增强HVO42−离子提取方面具有最强的能力。根据孔隙效应,提取效率卓越是由于有机相中提取化合物的体积更大。提取化合物[Omim]2[HVO4]的体积大于[Omim]Cl、[Omim]Br和[Omim][BF4],因为HVO42−是四种阴离子中体积最大的,导致其与[Omim]Cl、[Omim]Br和[Omim][BF4]相比具有更小的晶格能。异号离子之间较低的相互作用导致有机相中形成的提取化合物更为稳定,进而提高了提取效率。由于[Omim]Cl和[Omim]2[HVO4]之间的晶格能差异最大,在提取过程中离子交换更为彻底,因此可以实现V(Ⅴ)的最佳提取效率。
3.3 季铵盐类功能性离子液体萃钒机理
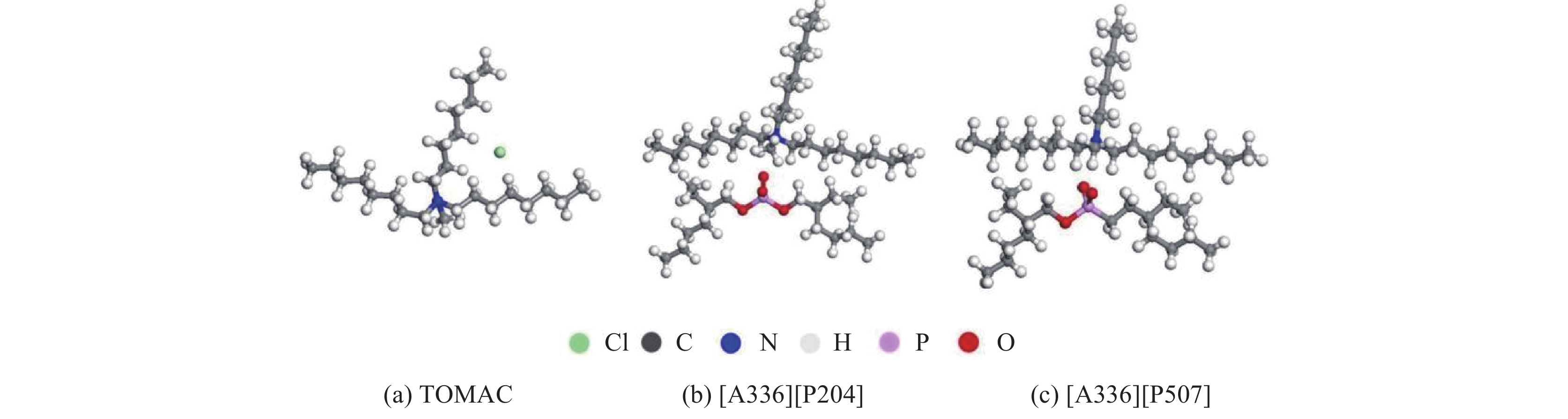
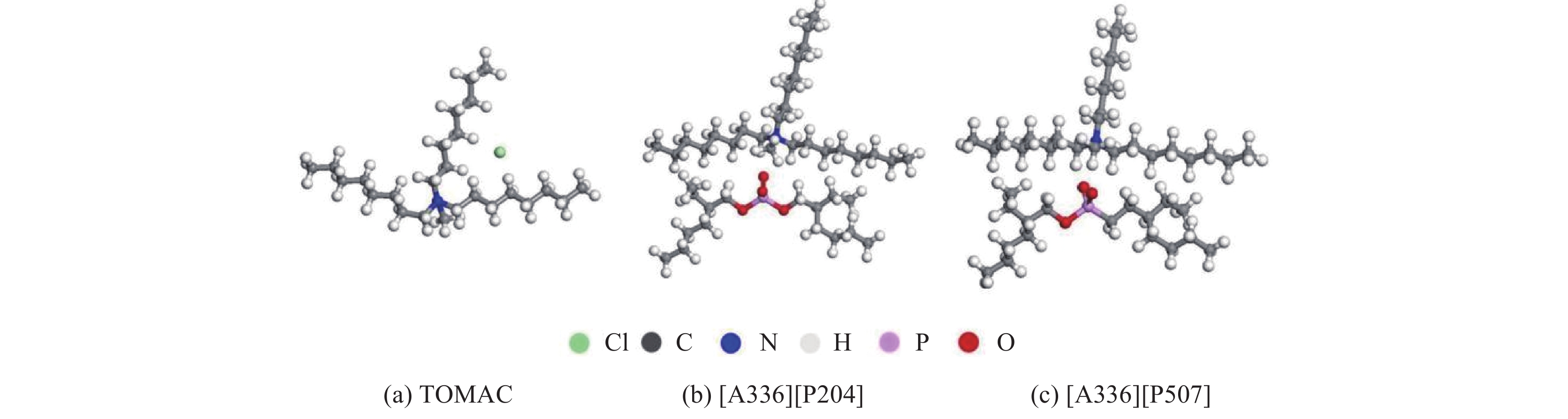
常见的季铵盐类离子液体有TOMAC、[A336][P204]、[A336][P507],其可能的分子模型如图5所示。
3.3.1 TOMAC萃钒动力学
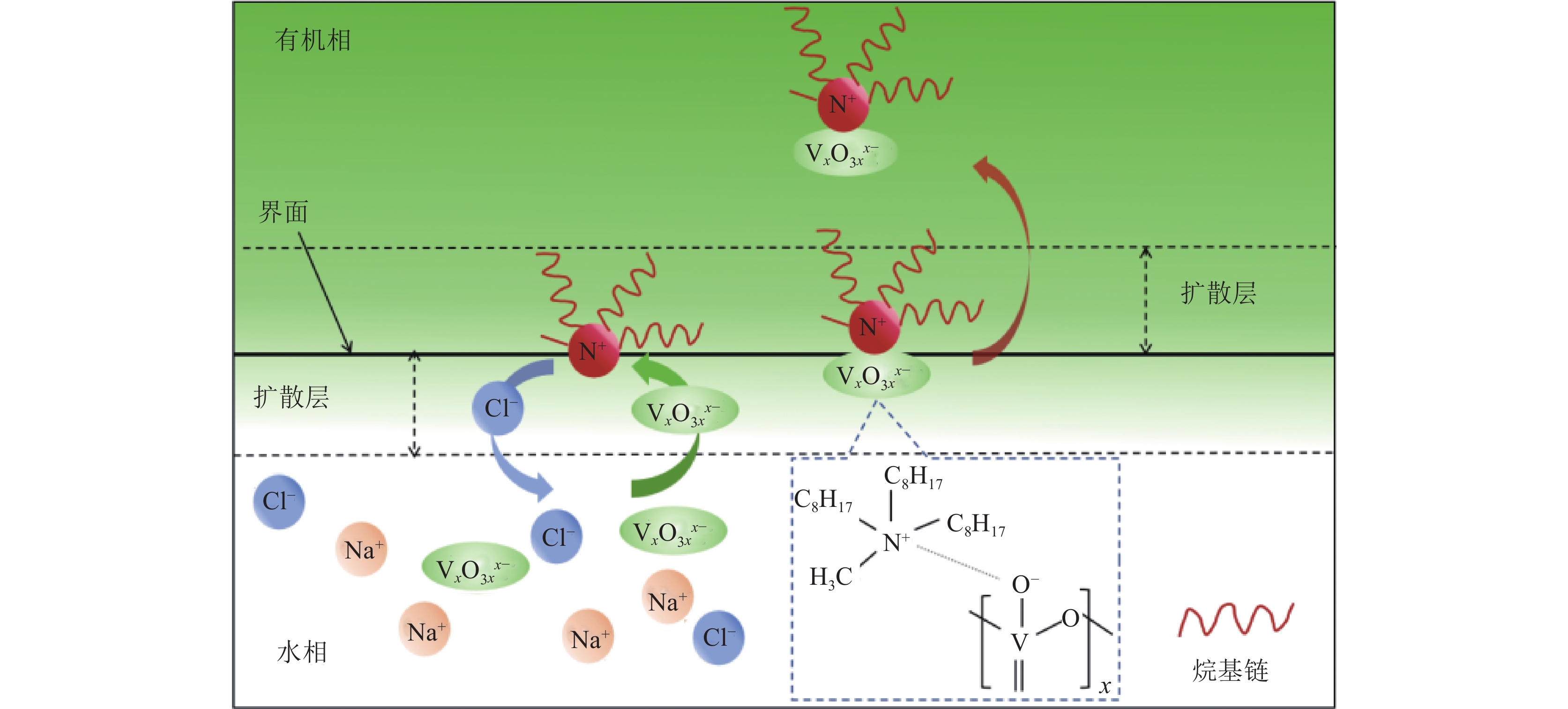
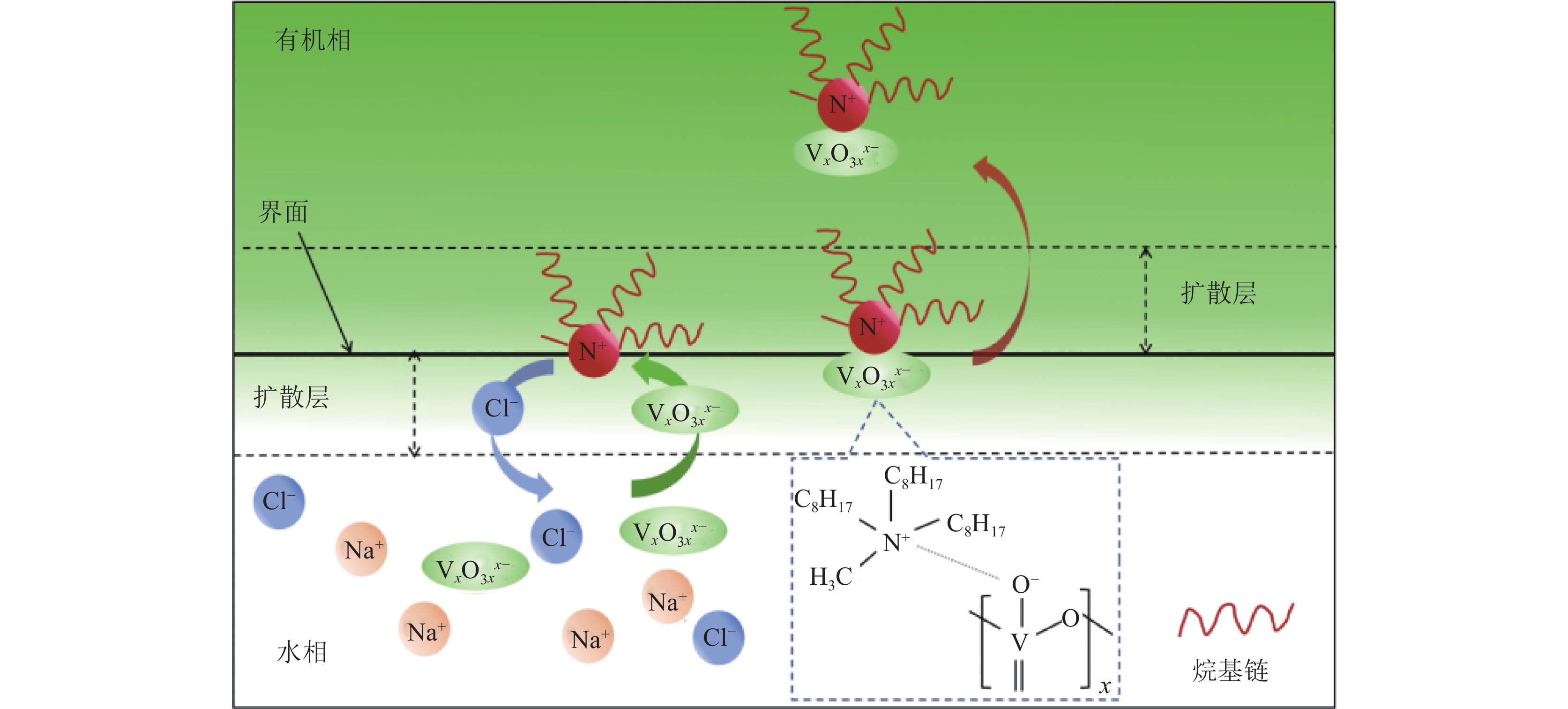
TOMAC对钒的萃取工艺如图6所示,该过程可分为三个关键步骤。首先,钒从水溶液中扩散至水-有机相界面,这是由于钒离子在两相之间的浓度梯度驱动下的分配过程。随后,钒在水-有机相界面被甲基三辛基氯化铵捕获,形成TOMAC-钒络合物。在这一步骤中,甲基三辛基氯化铵作为提取剂,通过钒离子与其结合,实现了钒的靶向提取。最后,TOMAC-钒络合物扩散到有机相中,形成稳定的体系[53]。通过这三个步骤,实现了钒从水溶液到有机相的高效转移和提取,为后续的分离和分析提供了基础。假如扩散被消除,萃取过程由捕获过程控制,假设捕获过程为一阶,萃取动力学微分方程可以表示为式(9)。
 图 6 TOMAC萃钒原理[53]Figure 6. Schematic illustration of the mechanism of vanadium extraction by TOMAC
图 6 TOMAC萃钒原理[53]Figure 6. Schematic illustration of the mechanism of vanadium extraction by TOMAC$$ r_{\mathrm{extr}}=\mathrm{d} c / \mathrm{d} t=k a\left(c-c_{\mathrm{e}}\right) $$ (9) 式中,rextr为萃取速率,mol /(L·s);t为萃取时间,s;c为时间t时水相中的钒浓度,mol/L;ce为水相中的平衡钒浓度,mol/L;k为体积萃取速率系数,s−1;a为比表面积,m2/m3,是水-有机相界面积与水相体积的比值。
水相中钒浓度与萃取时间的关系如方程(9)所示。
$$ c^*=1-\left(1-c_{\mathrm{e}}{ }^*\right)[1-\exp (-k a t)] $$ (10) 式中,c*为c / c0,c0为水相中初始钒浓度,mol/L。
Bal[54]表明TOMAC在较宽的pH范围内对钒(Ⅴ)的萃取效率较高(2<pH<9),并且所提取的物种均为阴离子型多金属氧酸盐:H2V10O284− (2<pH<5),HV10O285− (5<pH<7),V4O124− (7<pH<10)。TOMAC中的阳离子部分与溶液中游离的阴离子发生络合反应,生成含钒的有机络合物,从而达到净化富集酸浸液中钒的效果,该萃取反应方程式如下:
$$ {\begin{split} &4 \mathrm{CH}_3 \mathrm{NR}_3{ }^++\mathrm{H}_2 \mathrm{V}_{10} \mathrm{O}_{28}^{4-} \rightarrow\left(\mathrm{CH}_3 \mathrm{NR}_3\right)_4\mathrm{H}_2 \mathrm{V}_{10} \mathrm{O}_{28} \\ & (2<\mathrm{pH}<5) \end{split} } $$ (11) $$ {\begin{split} &5 \mathrm{CH}_3 \mathrm{NR}_3{ }^++\mathrm{HV}_{10} \mathrm{O}_{28}{ }^{5-} \rightarrow\left(\mathrm{CH}_3 \mathrm{NR}_3\right)_5 \mathrm{HV}_{10} \mathrm{O}_{28} \\ & (5<\mathrm{pH}<7) \end{split} } $$ (12) $$ \begin{split} &4 \mathrm{CH}_3 \mathrm{NR}_3{ }^++\mathrm{V}_4 \mathrm{O}_{12}{ }^{4-} \rightarrow\left(\mathrm{CH}_3 \mathrm{NR}_3\right)_4 \mathrm{V}_4 \mathrm{O}_{12} \\ & (7<\mathrm{pH}<10) \end{split} $$ (13) 3.3.2 [A336][P204]和[A336][P507]萃钒机理
[A336][P204]和[A336][P507]为常见的两种用于萃取钒的季铵盐类离子液体,它们的萃取机理相似。以[A336][P507]为例,其P═O和V═O之间的作用力使得V(Ⅳ)以VO2+和VOSO4形态被[A336][P507]萃取。其萃取机理为中性络合,其过程还伴随着提取水相中的H+和SO42−,依据萃合物电中性的原则,萃合物的化学式可表示为[VO2+]·[A336][P507]x·[(1+y/2)SO42−]。离子液体中存在很强的离子对相互作用,包括静电相互作用、范德华力和诱导相互作用等。这些相互作用均有助于提升[A336]+、[P507]−与V(Ⅳ)萃合物的稳定性,[A336][P507]对V(Ⅳ)的萃取表现出内协同作用,P═O与V═O之间形成较强的作用力,这也是[A336][P507]可以高效靶向性萃取V(Ⅳ)的原因[55]。
4. 功能性离子液体靶向萃钒的应用
爱沙尼亚的研究表明,采用[bmim]PF6或[bmim]Cl-AlCl3作为萃取剂,相较于传统溶剂,其产量增幅超过十倍,显著提升了萃取效率。同时,位于英国北爱尔兰的Queen’s大学正积极研究离子液体在油页岩萃取中的应用,这些离子液体的可循环性为其在环保与经济性方面带来了双重优势[56]。此外,Hu Qiaoyu等[51]深入研究了多种纯咪唑基疏水离子液体在V(Ⅴ)与Cr(Ⅵ)萃取过程中的表现。特别地,[C8mim][PF6]展现出了对V(Ⅴ)的高效萃取能力,而对Cr(Ⅵ)的萃取效果则相对有限。这一发现为从酸性废水中有效分离V(Ⅴ)与Cr(Ⅵ)提供了有力的工具,显著增强了分离效果,并符合当前对环保与资源回收的高标准要求。
He Jingui等[52]研究表明,辛基咪唑离子液体,如[Omim]Cl、[Omim]Br和[Omim][BF4]可用作V(Ⅴ)的萃取剂。在提取条件为平衡时间60 s、提取温度25 ℃时,[Omim]Cl、[Omim]Br和[Omim][BF4]的萃钒率分别达到97.93%、96.59%和87.01%。
TOMAC作为一种典型的季铵盐型离子液体,其核心作用机制在于其阴离子Cl−与待萃取基团间发生的交换反应。该离子液体在广泛的pH条件范围内均展现出对钒的高效萃取能力。罗大双[48]的研究聚焦于云母型钒页岩,经焙烧-酸浸处理后所得的多杂质含钒酸浸液中,TOMAC被选定为关键萃取剂。在优化的萃取工艺参数下,TOMAC对钒的萃取效率显著超越酸浸液中其他主要杂质离子,达到了93.18%的高水平。进一步分析显示,在此萃取体系中,钒与Fe、Al、Mg、K、P、Ca等杂质的分离系数分别高达
2434.9 、326.2、993.9、1075.0 、792.4及778.8,通过两级逆流萃取后,钒的萃取率达到98%,这一结果有力证明了TOMAC在萃取过程中能够极为有效地将钒与酸浸液中的多种杂质离子分离开来,体现了其卓越的萃取效率和强大的选择性,特别适用于从复杂酸浸液中高效提取钒元素。其反应方程式见式(14)。$$ 4\left(\mathrm{CH}_3 \mathrm{NR}_3\right)^++\mathrm{H}_2 \mathrm{V}_{10} \mathrm{O}_{28}{ }^{4-} \rightarrow\left[\left(\mathrm{CH}_3 \mathrm{NR}_3\right)_4 \mathrm{H}_2 \mathrm{V}_{10} \mathrm{O}_{28}\right] $$ (14) Wang等[57]使用TOMAC溶解于磺化煤油,成功实现了从钨酸铵水溶液中完全回收钒(Ⅴ)。建立了甲基TOMAC在磺化煤油中分离钒(Ⅴ)和铝(Ⅲ)的流程。结果表明,在初始pH为0.78、有机相与水相体积比为1:2、离子液体浓度40%、平衡时间为3 min时,钒的萃取率达到99.06%,铝的萃取率仅为7.95%。因此,分离因子β(V(Ⅴ)/Al(Ⅲ))为
1221 。TOMAC从页岩草酸浸出液中萃取钒(Ⅴ)的机理是VO(C2O4)22−阴离子与IL的氯离子之间的阴离子交换,其反应方程式见式(15)。与含有无机酸的体系相比,它不需要控制pH,可以更快地提取钒。$$ 2\left[\mathrm{R}_4 \mathrm{NCl}\right] + \mathrm{VO}\left(\mathrm{C}_2 \mathrm{O}_4\right)_2{ }^{2-} = \left[\left(\mathrm{R}_4 \mathrm{N}\right)_2 \mathrm{VO}\left(\mathrm{C}_2 \mathrm{O}_4\right)_2\right] + 2 \mathrm{Cl}^- $$ (15) Zhao Junmei[58]采用了一种创新的策略,即通过组合离子液体三辛基甲基硝酸铵(简称[A336][NO3])与有机酸化伯胺N1923(表示为[RNH3][NO3])的混合物,作为从含有Cr(Ⅵ)的废水中提取V(Ⅴ)的高效萃取剂。研究揭示,此混合物对V(Ⅴ)的萃取展现出显著的协同效应。在pH值为9.0,两者共同作用下,V(Ⅴ)的萃取分配比为630.4,V/Cr的分离系数达到了35,表明对V(Ⅴ)的提取具有高效选择性。此外,NO3−与V4O124−(或V3O93−)之间发生的阴离子交换机制有效抑制了有机阳离子-配体向水相中的释放,确保了萃取过程的可持续性。
贾蓝波的研究[55]则聚焦于[A336][P507]萃取体系,在特定的萃取条件下(包括使用0.5 mol/L NaOH调节酸浸液至pH=1.40,有机相组成为0.5 mol/L [A336][P507]-10% TBP-260#溶剂油,并采用O/A比为1:1进行3 min的萃取操作)实现了对钒、铁、铬的高选择性分离。具体而言,钒的萃取率高达98.53%,而铁和铬的萃取率则相对较低,分别为8.46%和5.46%,这充分证明了[A336][P507]体系对V(Ⅳ)具有强大的萃取能力及高选择性,且在此萃取过程中,有机相保持了良好的稳定性。
根据提供的参考文献和研究结果,对以下六种萃取剂([bmim]PF6、[C8mim][PF6]、[Omim]Cl、TOMAC、[A336][P507])对钒的萃取进行对比,结果如表3所示。
离子液体 钒的萃取率/% 杂质元素分离效果 萃取机理 [C8mim][PF6] 高(>90) 对Cr(VI)的选择性较低 阴离子交换:HV10O273−与PF6−之间的交换 [Omim]Cl 97.93 杂质分离效果好 阴离子交换:Cl−与HVO42−的交换 [Omim]Br 96.59 杂质分离效果好 阴离子交换:Br−与HVO42−的交换 [Omim][BF4] 87.01 杂质分离效果好 阴离子交换:BF4−与HVO42−的交换 TOMAC 98 对Fe、Al、Mg、K、P、Ca的分离系数高 阴离子交换:Cl−与多钒酸根离子的交换 [A336][NO3]或[RNH3][NO3] 高 V/Cr分离系数为35 阴离子交换:NO3−与V4O124− (或V3O93−)之间的交换 [bmim]PF6或
[bmim]Cl-AlCl3高 阴离子交换:PF6−或Cl−与钒离子之间的交换 [A336][P507] 98.53 对铁(8.46%)、铬(5.46%)的萃取率低 中性络合:P=O与V=O之间的作用力,
伴随H+和SO42−的提取[A336][P204] 77.27 杂质分离效果好,相分离快速,但有机相下层
有少量白色胶状物类似[A336][P507],中性络合 [bmim]PF6对钒的萃取率远高于传统溶剂,在萃取过程中可以实现钒和其他主要杂质离子(如Fe、Al、Mg、K、P、Ca等)的分离。这表明[bmim]PF6具有高效的萃取性能,但其所需温度较高,能耗较大。[C8mim][PF6]对钒的萃取作用较弱,但对V(Ⅴ)的特异性较强,这意味着[C8mim][PF6]在分离酸性废水中的V(Ⅴ)方面具有显著的效果。[Omim]Cl作为萃取剂可以用于钒(Ⅴ)的萃取,且在适当的提取条件下,钒的萃取率可以达到较高水平。总的来看,咪唑类和季铵盐类离子液体在实现工业化应用方面仍需不断努力,仍有很长的发展道路要走。TOMAC在萃取钒效果和选择性方面表现出色,对杂质离子的去除能力较强,这表明其对于酸浸液中的钒具有高效性和强的选择性,但其市价昂贵,且用量大。[A336][P507]对V(Ⅳ)的萃取效果强且选择性高,对杂质离子的去除效果明显但其在成本、大规模应用等方面仍面临一些挑战。
5. 结语与展望
1)离子液体具有低熔点、高热稳定性、低挥发度等物理化学性质,其可作为环境友好型试剂替代传统挥发性有机试剂,在提钒领域具有广阔的应用前景。
2)在含钒溶液中,钒主要以V(Ⅳ)和V(Ⅴ)两种价态存在。[Cnmim][PF6]主要通过阴离子交换过程萃取V(Ⅴ),而[Omim][X](X=Cl−、Br−、BF4−)也为类似的阴离子交换机理提取V(Ⅴ)。此外,在季铵盐类离子液体中,TOMAC中的阳离子与溶液中游离的阴离子形成络合物,实现了含钒有机络合物的生成。[A336][P204]和[A336][P507]利用P═O和V═O之间的作用力,中性络合机理萃取V(Ⅳ)的VO2+和VOSO4形态,且[A336][P507]展现出高效选择性萃取V(Ⅳ)的能力,其内协同作用加强了V(Ⅳ)的稳定性。特异性离子液体靶向萃取钒的机理为选取合适的萃取剂提供了重要参考。这些离子液体展现出高选择性和强靶向性,利用特定作用力形成稳定的络合物,提升了钒的萃取效率。此外,这种萃取方式对环境友好,为绿色萃钒提供了新的路径。
3)未来的研究可以进一步探索离子液体在钒萃取中的应用,包括但不限于以下几个方面:①优化萃取剂结构:在咪唑和季铵盐类离子液体的基础上,结合二者优点设计和合成新型离子液体,改善钒的选择性和提取效率。②萃取剂回收和循环利用:开发高效的离子液体回收和再利用技术,减少成本和环境影响。③工业应用推广:将离子液体萃取技术应用于实际的工业生产中,进一步验证其可行性和经济性。
-
图 1 合成离子液体的方法流程[33]
Figure 1. Flow chart for the synthesis of ionic liquids
图 2 [Omim]Br的反应流程[41]
Figure 2. The reaction flow chart of [Omim]Br
图 6 TOMAC萃钒原理[53]
Figure 6. Schematic illustration of the mechanism of vanadium extraction by TOMAC
表 1 常用于萃钒的离子液体类型
Table 1. Types of ionic liquids commonly used in vanadium extraction
离子液体类型 离子液体类别名称及相关化学式 阳离子 咪唑 
苯并咪唑盐 
铵 
吡啶盐 
阴离子 六氟磷酸根( Ⅴ ) 
四氟硼酸根 
卤素阴离子 
醋酸盐 
硝酸盐 
2,2,2 -三氟乙酸 
双离子 咪唑类
([C6mim][PF6])
([C8mim][PF6])C12H23ClN2
([Omim]Cl)
C12H23BrN2
([Omim]Br)
C12H23BF4N2
([Omim][BF4])
([Bmim]PF6)
Bmim Cl-AlCl3季铵盐类 
C25H54ClN
[A336][P204]
[A336][P507]表 2 常见咪唑类和季铵盐类离子液体物理化学性质
Table 2. Physicochemical properties of imidazolium and quaternary ammonium ionic liquids
离子液体类型 名称 熔点/ ℃ 体积密度
(20 ℃)/(g·cm−3)分子量 咪唑类 [C6mim][PF6] −73.5 1.3045 312.24 [C8mim][PF6] −70 1.2345 340.29 [Omim]Cl 12 1.01 230.78 [Omim]Br −61.9 1.17 275.23 [Omim][BF4] −80 1.09 282.13 [bmim]PF6 6.5 1.38 284.18 季铵盐类 C25H54ClN −20 0.884 404.16 离子液体 钒的萃取率/% 杂质元素分离效果 萃取机理 [C8mim][PF6] 高(>90) 对Cr(VI)的选择性较低 阴离子交换:HV10O273−与PF6−之间的交换 [Omim]Cl 97.93 杂质分离效果好 阴离子交换:Cl−与HVO42−的交换 [Omim]Br 96.59 杂质分离效果好 阴离子交换:Br−与HVO42−的交换 [Omim][BF4] 87.01 杂质分离效果好 阴离子交换:BF4−与HVO42−的交换 TOMAC 98 对Fe、Al、Mg、K、P、Ca的分离系数高 阴离子交换:Cl−与多钒酸根离子的交换 [A336][NO3]或[RNH3][NO3] 高 V/Cr分离系数为35 阴离子交换:NO3−与V4O124− (或V3O93−)之间的交换 [bmim]PF6或
[bmim]Cl-AlCl3高 阴离子交换:PF6−或Cl−与钒离子之间的交换 [A336][P507] 98.53 对铁(8.46%)、铬(5.46%)的萃取率低 中性络合:P=O与V=O之间的作用力,
伴随H+和SO42−的提取[A336][P204] 77.27 杂质分离效果好,相分离快速,但有机相下层
有少量白色胶状物类似[A336][P507],中性络合 -
[1] Zhu Siqin, Ye Guohua, Kang Xuanxiong, et al. Research progress on the treatment of acid leaching vanadium wastewater from vanadium-bearing shale[J]. Water Treatment Technology, 2024,50(5):1-7. (朱思琴, 叶国华, 亢选雄, 等. 含钒页岩酸浸提钒废水处置的研究进展[J]. 水处理技术, 2024,50(5):1-7.Zhu Siqin, Ye Guohua, Kang Xuanxiong, et al. Research progress on the treatment of acid leaching vanadium wastewater from vanadium-bearing shale[J]. Water Treatment Technology, 2024, 50(5): 1-7. [2] Wu You, Chen Donghui, Liu Wuhan, et al. Global vanadium industry development report 2022[J]. Iron Steel Vanadium Titanium, 2023,44(6):1-8. (吴优, 陈东辉, 刘武汉, 等. 2022年全球钒工业发展报告[J]. 钢铁钒钛, 2023,44(6):1-8.Wu You, Chen Donghui, Liu Wuhan, et al. Global vanadium industry development report 2022[J]. Iron Steel Vanadium Titanium, 2023, 44(6): 1-8. [3] Zhang Yimin, Xue Nannan, Zheng Qiushi, et al. Research status and development of the whole industry chain utilization of vanadium shale resources[J]. China Nonferrous Metallurgy, 2023,52(5):2-17. (张一敏, 薛楠楠, 郑秋实, 等. 钒页岩资源全产业链利用研究现状及发展[J]. 中国有色冶金, 2023,52(5):2-17.Zhang Yimin, Xue Nannan, Zheng Qiushi, et al. Research status and development of the whole industry chain utilization of vanadium shale resources[J]. China Nonferrous Metallurgy, 2023, 52(5): 2-17. [4] Zhang Yimin, Xue Nannan, Liu Tao, et al. Vanadium shale wet green extraction technology[J]. Journal of China University of Mining and Technology, 2022,51(3):520-531. (张一敏, 薛楠楠, 刘涛, 等. 钒页岩全湿法绿色提取技术[J]. 中国矿业大学学报, 2022,51(3):520-531.Zhang Yimin, Xue Nannan, Liu Tao, et al. Vanadium shale wet green extraction technology[J]. Journal of China University of Mining and Technology, 2022, 51(3): 520-531. [5] Jia Li, Zhang Yimin, Liu Tao, et al. A methodology for assessing cleaner production in the vanadium extraction industry[J]. Journal of Cleaner Production, 2014,84:598-605. doi: 10.1016/j.jclepro.2013.05.016 [6] Zhang Yimin, Bao Shenxu, Liu Tao, et al. The technology of extracting vanadium from stone coal in China: History, current status and future prospects[J]. Hydrometallurgy, 2011,109:116-124. doi: 10.1016/j.hydromet.2011.06.002 [7] Xiang Xinyue, Ye Guohua, Zhu Siqin, et al. Research progress on extraction of vanadium from acid leaching solution containing vanadium[J]. Protection and Utilization of Mineral Resources, 2023,43(5):170-178. (项新月, 叶国华, 朱思琴, 等. 从含钒酸浸液中萃取提钒的研究进展[J]. 矿产保护与利用, 2023,43(5):170-178.Xiang Xinyue, Ye Guohua, Zhu Siqin, et al. Research progress on extraction of vanadium from acid leaching solution containing vanadium[J]. Protection and Utilization of Mineral Resources, 2023, 43(5): 170-178. [8] Lai Yongchuan, Yang Xinlong, Sun Jianzhi, et al. Research status of selective extraction of vanadium from acid leaching solution of vanadium-bearing shale[J]. Rare Metals, 2022,46(1):109-119. (赖永传, 杨鑫龙, 孙建之, 等. 含钒页岩酸浸液中钒的选择性萃取研究现状[J]. 稀有金属, 2022,46(1):109-119.Lai Yongchuan, Yang Xinlong, Sun Jianzhi, et al. Research status of selective extraction of vanadium from acid leaching solution of vanadium-bearing shale[J]. Rare Metals, 2022, 46(1): 109-119. [9] Zhang Yimin, Bao Shenxu. Present situation and prospect of purification and enrichment technology for vanadium-containing leaching solution[J]. Metal Mines, 2016(7):64-70. (张一敏, 包申旭. 含钒浸出液净化富集技术现状及前景[J]. 金属矿山, 2016(7):64-70.Zhang Yimin, Bao Shenxu. Present situation and prospect of purification and enrichment technology for vanadium-containing leaching solution[J]. Metal Mines, 2016(7): 64-70. [10] Zhang Ying, Zhang Ting’an. Research progress of vanadium extraction from vanadium-containing leaching solution by solvent extraction[J]. Nonferrous Metal Science and Engineering, 2017,8(5):14-20. (张莹, 张廷安. 溶剂萃取法从含钒浸出液中提钒的研究进展[J]. 有色金属科学与工程, 2017,8(5):14-20.Zhang Ying, Zhang Ting’an. Research progress of vanadium extraction from vanadium-containing leaching solution by solvent extraction[J]. Nonferrous Metal Science and Engineering, 2017, 8(5): 14-20. [11] Dan Weijie, Xiao Liansheng, Zhang Guiqing, et al. Extraction of chromium (Ⅲ) from iron (Ⅱ) by extraction[J]. Nonferrous Metal Science and Engineering, 2017,8(3):35-41. (淡维杰, 肖连生, 张贵清, 等. 萃取法提取铬(Ⅲ)分离铁(Ⅱ)的研究[J]. 有色金属科学与工程, 2017,8(3):35-41.Dan Weijie, Xiao Liansheng, Zhang Guiqing, et al. Extraction of chromium (Ⅲ) from iron (Ⅱ) by extraction[J]. Nonferrous Metal Science and Engineering, 2017, 8(3): 35-41. [12] Xu Liang. Extraction of vanadium from high concentration sulfuric acid solution[D]. Changsha: Central South University, 2013. (许亮. 从高浓度的硫酸溶液中萃取钒的研究[D]. 长沙: 中南大学, 2013.Xu Liang. Extraction of vanadium from high concentration sulfuric acid solution[D]. Changsha: Central South University, 2013. [13] Chen Ziyang, Ye Guohua, Zuo Qi, et al. Structure-activity relationship of organic amine extractants and research progress of vanadium extraction[J]. Iron Steel Vanadium Titanium, 2020,41(3):8-15. (陈子杨, 叶国华, 左琪, 等. 有机胺类萃取剂构效关系及其萃钒的研究进展[J]. 钢铁钒钛, 2020,41(3):8-15.Chen Ziyang, Ye Guohua, Zuo Qi, et al. Structure-activity relationship of organic amine extractants and research progress of vanadium extraction[J]. Iron Steel Vanadium Titanium, 2020, 41(3): 8-15. [14] Tang Yue, Ye Guohua, Zhang Hao, et al. Solvent extraction of vanadium with D2EHPA from aqueous leachate of stone coal after low–temperature sulfation roasting[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022,650:129584. doi: 10.1016/j.colsurfa.2022.129584 [15] Sun Xiaoqi, Ji Yang, Hu Fengchun, et al. The inner synergistic effect of bifunctional ionic liquid extractant for solvent extraction[J]. Talanta, 2010,81:1877-1883. doi: 10.1016/j.talanta.2010.03.041 [16] Wang Wei, Chen Ji, Liu Hongzhao, et al. Research progress of functional ionic liquids in metal extraction and separation[J]. Application Chemistry, 2015,32(7):733-742. (王威, 陈继, 刘红召, 等. 功能性离子液体在金属萃取分离中的研究进展[J]. 应用化学, 2015,32(7):733-742.Wang Wei, Chen Ji, Liu Hongzhao, et al. Research progress of functional ionic liquids in metal extraction and separation[J]. Application Chemistry, 2015, 32(7): 733-742. [17] Tang Yue, Ye Guohua, Hu Yujie, et al. Application status and development trend of ionic liquids in extraction and separation[J]. Mining and Metallurgy, 2021,30(6):54-62. (唐悦, 叶国华, 胡渝杰, 等. 离子液体在萃取分离中的应用现状与发展趋势[J]. 矿冶, 2021,30(6):54-62.Tang Yue, Ye Guohua, Hu Yujie, et al. Application status and development trend of ionic liquids in extraction and separation[J]. Mining and Metallurgy, 2021, 30(6): 54-62. [18] Yu Chen, Bao Shenxu, Zhang Yimin, et al. Separation and adsorption of V(Ⅴ) from canadium-containing solution by TOMAC-impregnated resins[J]. Chemical Engineering Research and Design, 2021,174:405-413. doi: 10.1016/j.cherd.2021.08.019 [19] Maria Atanassova. Solvent extraction of metallic species in ionic liquids: an overview of F-ions[J]. Journal of Chemical Technology & Metallurgy, 2021,56(3):433-466. [20] Pavel A Yudaev, Evgeniy M Chistyakov. Ionic liquids as components of systems for metal extraction[J]. Chem. Engineering, 2022,6(1):6. [21] Ling Yunwang, Qing Jieguo, Man Seunglee. Recent advances in metal extraction improvement: Mixture systems consisting of ionic liquid and molecular extractant[J]. Separation and Purification Technology, 2019,210:292-303. doi: 10.1016/j.seppur.2018.08.016 [22] Wu Yunyan, Zhao Yanfei, Li Ruipeng, et al. Ionic liquids promoted Ru / C catalyzed N-formylation of organic amines with CO2 / H2[J]. Chinese Science: Chemistry, 2020,50(2):299-305. (吴云雁, 赵燕飞, 李瑞鹏, 等. 离子液体促进 Ru/C 催化有机胺与 CO2/H2 氮甲酰化反应研究[J]. 中国科学: 化学, 2020,50(2):299-305.Wu Yunyan, Zhao Yanfei, Li Ruipeng, et al. Ionic liquids promoted Ru / C catalyzed N-formylation of organic amines with CO2 / H2[J]. Chinese Science: Chemistry, 2020, 50(2): 299-305. [23] Camiel H C Janssen. Prevailing mechanisms in pseudo-protic ionic liquid metal extractions[J]. Journal of Molecular Liquids, 2020,304:112738 . doi: 10.1016/j.molliq.2020.112738 [24] Sun Qian, Liu Yuanlan, Lu Jiaxing. Application of ionic liquids in electrochemistry[J]. Chemical Bulletin, 2003,66(2):113-114. (孙茜, 刘元兰, 陆嘉星. 离子液体在电化学中的应用[J]. 化学通报, 2003,66(2):113-114.Sun Qian, Liu Yuanlan, Lu Jiaxing. Application of ionic liquids in electrochemistry[J]. Chemical Bulletin, 2003, 66(2): 113-114. [25] Tsai Wanyu, Come J, Zhao Wei, et al. Hysteretic order-disorder transitions of ionic liquid double layer structure on graphite[J]. Nano Energy, 2019, 60: 886-893. [26] Sun Yinshi, Liu Zhengbo, Wang Jianhua, et al. Aqueous ionic liquid based ultrasonic assisted extraction of four acetophenones from the Chinese medicinal plant Cynanchum bungei Decne[J]. Ultrasonics Sonochemistry, 2013,20(1):180-186. doi: 10.1016/j.ultsonch.2012.07.002 [27] Hitesh Sehrawat, Neeraj Kumar, Ravi Tomar, et al. Synthesis and characterization of novel 1, 3benzodioxole tagged noscapine based ionic liquids with in silico and in vitro cytotoxicity analysis on HeLa cells[J]. Journal of Molecular Liquids, 2020,302:112525. doi: 10.1016/j.molliq.2020.112525 [28] Liu Mengying, Che Jianing, Wu Weihong, et al. Extraction of Cu2+ from aqueous solution by functional ionic liquids: Experiment and theory[J]. Acta Chimica Sinica, 2015(2):116-125. (刘梦莹, 车佳宁, 吴蔚闳, 等. 功能性离子液体萃取水溶液中 Cu2+: 试验与理论[J]. 化学学报, 2015(2):116-125.Liu Mengying, Che Jianing, Wu Weihong, et al. Extraction of Cu2+ from aqueous solution by functional ionic liquids: Experiment and theory[J]. Acta Chimica Sinica, 2015(2): 116-125. [29] Shi Jiahua, Sun Xun, Yang Chunhe, et al. Research progress of ionic liquids[J]. Chemical Bulletin, 2002,65(4):243250. (石家华, 孙逊, 杨春和, 等. 离子液体研究进展[J]. 化学通报, 2002,65(4):243250.Shi Jiahua, Sun Xun, Yang Chunhe, et al. Research progress of ionic liquids[J]. Chemical Bulletin, 2002, 65(4): 243250. [30] Shaukat Ali Mazari, Ahsan Raza Siyal, Nadeem Hussain Solangi, et al. Prediction of thermo-physical properties of 1-Butyl-3-methylimidazolium hexafluorophosphate for CO2 capture using machine learning models[J]. Journal of Molecular Liquids, 2021,327:114785. doi: 10.1016/j.molliq.2020.114785 [31] Dmitri Nikitin, Sergei Preis, Niina Dulova. Degradation of imidazolium-based ionic liquids by UV photolysis and pulsed corona discharge: The effect of persulfates addition[J]. Separation and Purification Technology, 2024,344:127235. doi: 10.1016/j.seppur.2024.127235 [32] Rajni Ratti. Ionic liquids: synthesis and applications in catalysis[J]. Adv. Chem., 2014(9):729842. [33] Sandip K Singh, Anthony W Savoy. Ionic liquids synthesis and applications: An overview[J]. Journal of Molecular Liquids, 2020,297:112038. doi: 10.1016/j.molliq.2019.112038 [34] Joan Fuller, Richard T Carlin, Robert A Osteryoung. The room temperature ionic liquid 1‐Ethyl‐3‐methylimidazolium tetrafluoroborate: Electrochemical couples and physical properties[J]. Journal of the Electrochemical Society, 1997,144:3881-3885. doi: 10.1149/1.1838106 [35] Peter Wasserscheid, Roy van Hal, Andreas Bösmann. 1-n-Butyl-3-methylimidazolium ([bmim]) octylsulfate—an even “greener” ionic liquid[J]. Green Chemistry, 2002(4): 400-404. [36] Richard P Swatloski, Scott K Spear, Holbrey J D. Dissolution of cellose with ionic liquids[J]. Journal of the American Chemical Society, 2002,124(18):4974-4975. doi: 10.1021/ja025790m [37] Chu Xuemei, Hu Yufeng, Li Jiguang, et al. Desulfurization of diesel fuel by extraction with [BF4]−-based ionic liquids[J]. Chinese Journal of Chemical Engineering, 2008,16(6):881-884. doi: 10.1016/S1004-9541(09)60010-0 [38] Li Jiguang, Hu Yufeng, Chu Hongda, et al. Thermodynamic properties of [C6mim] [PF6] and [C8mim] [PF6][J]. Journal of Natural Science, Heilongjiang University, 2009,26(5):674-678. (李吉广, 胡玉峰, 褚洪达, 等. 离子液体[C6mim][PF6]和[C8mim][PF6]的热力学性质研究[J]. 黑龙江大学自然科学学报, 2009,26(5):674-678.Li Jiguang, Hu Yufeng, Chu Hongda, et al. Thermodynamic properties of [C6mim] [PF6] and [C8mim] [PF6][J]. Journal of Natural Science, Heilongjiang University, 2009, 26(5): 674-678. [39] Wu Peiwen, Xun Suhang, Jiang Wei, et al. Research progress of ionic liquid reactive extraction fuel desulfurization[J]. Journal of Chemical Industry, 2021,72(1):276-291. (吴沛文, 荀苏杭, 蒋伟, 等. 离子液体反应型萃取燃油脱硫研究进展[J]. 化工学报, 2021,72(1):276-291.Wu Peiwen, Xun Suhang, Jiang Wei, et al. Research progress of ionic liquid reactive extraction fuel desulfurization[J]. Journal of Chemical Industry, 2021, 72(1): 276-291. [40] Peng Xuelian. Preparation of supported polyoxometalate-ionic liquid catalyst and its oxidative desulfurization performance[D]. Wuhan: Wuhan University of Engineering, 2023. (彭雪连. 负载型多金属氧酸盐-离子液体催化剂的制备及其氧化脱硫性能研究[D]. 武汉: 武汉工程大学, 2023.Peng Xuelian. Preparation of supported polyoxometalate-ionic liquid catalyst and its oxidative desulfurization performance[D]. Wuhan: Wuhan University of Engineering, 2023. [41] Sen Nirvik, Singh K K, Mukhopadhyay S. Solvent-free flow synthesis of [OMIM]Br in a microreactor[J]. Chemical Engineering and Processing - Process Intensification, 2021, 166: 108431. [42] Wang Jiaqi. Environmental impact assessment of ionic liquid gas separation process based on full life cycle[D]. Beijing: Beijing University of Technology, 2022. (王佳琪. 基于全生命周期的离子液体气体分离过程环境影响评价[D]. 北京: 北京工业大学, 2022.Wang Jiaqi. Environmental impact assessment of ionic liquid gas separation process based on full life cycle[D]. Beijing: Beijing University of Technology, 2022. [43] Wang Jiaqi, Dai Chengna, Yu Gangqiang, et al. Technology-environmental assessment of imidazolium ionic liquids for natural gas dehydration[J]. Environmental Engineering, 2022,40(11):199-210. (王佳琪, 代成娜, 于刚强, 等. 咪唑基离子液体用于天然气脱水过程中的技术-环境评价[J]. 环境工程, 2022,40(11):199-210.Wang Jiaqi, Dai Chengna, Yu Gangqiang, et al. Technology-environmental assessment of imidazolium ionic liquids for natural gas dehydration[J]. Environmental Engineering, 2022, 40(11): 199-210. [44] Zhang Nan, Deng Tianlong, Liu Mingming, et al. Synthesis and physicochemical properties of ionic liquids [Bmim]Br and [Bmim]PF6[J]. Tianjin: Journal of Tianjin University of Science and Technology, 2014,29(5):42-47. (张楠, 邓天龙, 刘明明, 等. 离子液体[Bmim]Br和[Bmim]PF6的合成及其物化性质研究[J]. 天津: 天津科技大学学报, 2014,29(5):42-47.Zhang Nan, Deng Tianlong, Liu Mingming, et al. Synthesis and physicochemical properties of ionic liquids [Bmim]Br and [Bmim]PF6[J]. Tianjin: Journal of Tianjin University of Science and Technology, 2014, 29(5): 42-47. [45] Swapnil A Padvi, Yogesh A Tayade, Yogesh B Wagh, et al. [Bmim]OH: An efficient catalyst for the synthesis of mono and bis spirooxindole derivatives in ethanol at room temperature[J]. Chinese Chemical Letters, 2016,27(5):714-720. doi: 10.1016/j.cclet.2016.01.016 [46] Fan Mingming, Zhang Pingbo. Activated carbon supported K2CO3 catalysts for transesterification of dimethyl carbonate with propyl alcohol[J]. Energy & Fuels, 2007,21(2):633-635. [47] Parida K, Dash S, Singha S. Structural properties and catalytic activity of Mn-MCM-41 mesoporous molecular sieves for single-step amination of benzene to aniline[J]. Applied Catalysis A-General, 2008,351(1):59-67. doi: 10.1016/j.apcata.2008.08.027 [48] Luo Dashuang. Study on the efficient selective extraction of vanadium from shale acid leaching solution by trioctylmethylammonium chloride[D]. Wuhan: Wuhan University of Science and Technology, 2021. (罗大双. 三辛基甲基氯化铵高效选择萃取页岩酸浸液中钒的研究[D]. 武汉: 武汉科技大学, 2021.Luo Dashuang. Study on the efficient selective extraction of vanadium from shale acid leaching solution by trioctylmethylammonium chloride[D]. Wuhan: Wuhan University of Science and Technology, 2021. [49] Hu Yibo, Zhang Yimin, Xue Nannan, et al. Nφ−pH diagrams and kinetics of V2O3 prepared by solution-phase hydrogen reduction[J]. Transactions of Nonferrous Metals Society of China, 2022,32(4):1290-1300. doi: 10.1016/S1003-6326(22)65874-6 [50] Gao Qiang. Experimental study on the synthesis method of iron vanadate[D]. Shenyang: Northeastern University, 2013. (高蔷. 钒酸铁合成方法的实验研究[D]. 沈阳: 东北大学, 2013.Gao Qiang. Experimental study on the synthesis method of iron vanadate[D]. Shenyang: Northeastern University, 2013. [51] Hu Qiaoyu, Zhao Junmei, Wang Fuchun, et al. Selective extraction of vanadium from chromium by pure [C8mim][PF6]: An anion exchange process[J]. Separation and Purification Technology, 2014,131:94-101. doi: 10.1016/j.seppur.2014.05.003 [52] He Jingui, Tao Wenju, Dong Guozhen. Study on extraction performance of vanadium (Ⅴ) from aqueous solution by octyl-imidazole ionic liquids extractants[J]. Metals, 2022,12:854. doi: 10.3390/met12050854 [53] Zhang Fengzhen, Zhang Huanhuan, Zhang Wei, et al. Kinetics of ultrasound-assisted solvent extraction of vanadium with methyltrioctylammonium chloride (MAC)[J]. Hydrometallurgy, 2023,221:106142. doi: 10.1016/j.hydromet.2023.106142 [54] Bal Y, Bal K E, Cote G. Kinetics of the alkaline stripping of vanadium(Ⅴ) previously extracted by Aliquat 336[J]. Minerals Engineering, 2002,15:377-379. doi: 10.1016/S0892-6875(02)00044-4 [55] Jia Lanbo. Experimental study on vanadium extraction from acid leaching solution of vanadium slag[D]. Tangshan: North China University of Science and Technology, 2022. (贾蓝波. 钒渣酸浸液萃取提钒试验研究[D]. 唐山: 华北理工大学, 2022.Jia Lanbo. Experimental study on vanadium extraction from acid leaching solution of vanadium slag[D]. Tangshan: North China University of Science and Technology, 2022. [56] Li Ruxiong, Wang Jianji. Synthesis and application of ionic liquids[J]. Chemical Reagents, 2001(4):211-215. (李汝雄, 王建基. 离子液体的合成与应用[J]. 化学试剂, 2001(4):211-215. doi: 10.3969/j.issn.0258-3283.2001.04.008Li Ruxiong, Wang Jianji. Synthesis and application of ionic liquids[J]. Chemical Reagents, 2001(4): 211-215. doi: 10.3969/j.issn.0258-3283.2001.04.008 [57] Wang Liupei, Zhang Guiqing, Guan Wenjuan. Complete removal of trace vanadium from ammonium tungstate solutions by solvent extraction[J]. Hydrometallurgy, 2018,179:268-273. doi: 10.1016/j.hydromet.2018.06.016 [58] Zhao Junmei, Hu Qiaoyu, Li Yingbo, et al. Efficient separation of vanadium from chromium by a novel ionic liquid-based synergistic extraction strategy[J]. Chemical Engineering Journal, 2015,264:487-496. doi: 10.1016/j.cej.2014.11.071 -





 下载:
下载:



 下载:
下载: