Preparation of titanium by hydrogenation and analysis of its energy
-
摘要: 以海绵钛为原料,在不同温度、不同时间下制备出氢化钛。根据重量变化分析其反应原理,用X射线衍射仪(XRD)进行结构分析,扫描电镜(SEM)进行形貌分析。结果显示:随反应温度升高,氢化钛质量增加率增大,脆性也变大。当温度高于500 ℃之后,质量增长率变化不大。热力学计算结果发现温度从300 ℃增加到700 ℃时,平衡常数显著下降,表明反应温度过高不利于反应进行。氢化过程的吸附能量计算表明其较优吸附位位于其中心点位。固溶能量分析表明氢原子更倾向于占据八面体间隙。Abstract: Titanium hydride was prepared from sponge titanium at different temperatures and time. Perform structural analysis using X-ray diffraction (XRD) and morphology analysis using scanning electron microscopy (SEM). The results show that with the increase of reaction temperature, the mass growth rate of titanium hydride increases, and the brittleness also increases. When the temperature exceeds 500 ℃, there is little change in the mass growth rate. The thermodynamic calculation results show that when the temperature increases from 300 ℃ to 700 ℃, the equilibrium constant significantly decreases, indicating that a high reaction temperature is not conducive to the reaction. The calculation of adsorption energy in the hydrogenation process shows that the optimal adsorption site is located at its central point. Solid solution energy analysis shows that hydrogen atoms tend to occupy octahedral gaps more.
-
Key words:
- titanium hydride /
- sponge titanium /
- reaction temperature /
- prepartion /
- characterization
-
0. 引言
吸氢与储氢性能是钛基合金的重要研究领域。钛具有良好的吸氢性能,可以吸收大量的氢气。这一特性使得钛在氢能源领域具有重要应用价值[1-2]。从结构上看,钛具有两种同素异构体,在882.5 ℃以下为密排六方结构(hcp)的α-Ti晶体,在882.5 ℃至熔点之间为体心立方结构(bcc)的β-Ti 晶体。钛的吸氢是将钛直接与氢气反应获得氢化钛,钛在吸氢时的温度一般不超过680 ℃。因此α-Ti的结构成为其吸氢与储氢性能的关键。目前,从试验角度分析吸氢的影响因素 [3-4]和理论计算角度分析吸氢的过程[5-6]均有研究。然而,试验分析和理论计算相结合,从温度与能量角度分析其吸氢过程研究较少。而温度与能量角度关系是了解其吸氢过程与透氢过程的关键。通过对钛的吸氢制备研究,有助于深入了解金属氢化物的氢吸附机制[7-8]。对钛的吸氢制备过程的能量分析可以评估该过程的能源效益和可持续性,提高氢能源的储存效率、推动金属氢化物储氢材料的研究与应用、优化制备工艺、评估环境影响和促进可持续发展。为此,笔者在这方面开展了探索。
1. 试验过程
1.1 样品制备
称量海绵钛颗粒加入石英舟中,将石英舟放入在管式炉中待加热。将氢气发生器通电打开,使氢气排出量达到300 mL/min后将通气管接入管式炉的一端,保持密封,将管式炉的另一端也接上通气管,保持密封。管式炉两端气管接好后通氢30 min,以确保排尽石英管中的残留的空气,调节管式炉的升温曲线,温度分别为400、450、500、550 ℃,保温时间30 min。保持炉内的氢气压力并持续通入氢气,使样品随炉冷却,得到氢化钛,称量氢化钛的质量并记录。
1.2 分析测试
海绵钛高温氢化反应会吸收氢气,从而使质量增加。根据海绵钛氢化原理可以计算出在理想情况下试验前后质量的变化率。采用重量分析方法,通过实际质量变化率与理论质量变化率的比较可以得到最佳的反应条件,其公式如式(1)(2)所示。
$$ \frac{2}{\mathrm{\mathit{x}}}\mathrm{Ti}(\mathrm{s})+\mathrm{H}_2(\mathrm{g})=\frac{2}{\mathit{\mathrm{\mathit{x}}}}\mathrm{TiH}_x(\mathrm{s})+\mathrm{\mathit{Q}}\mathit{ } $$ (1) $$ W=\frac{M_{\mathrm{H}_2}}{M\mathrm{_{Ti}}}=\frac{1.008\times2}{47.867}=4.2\text{%} $$ (2) 式中 x是与Ti反应的H的化学计量比;M为物质的分子量;W为钛金属的吸氢量。
样品形貌观察采用扫描电镜(VEGA‖XMH型),微观结构用X射线衍射仪(D8 ADVANCE)观察。
1.3 计算方法
采用基于密度泛函理论的第一原理计算软件materials studio的Adsorption Locator模块和CASTEP模块。为去除H2分子相互作用影响,设置2×2×1的超晶胞。表面计算中设置真空层的厚度为1.5 nm Locator 模块。力场Forcefield选择为Universal,设置最大吸附距离为0.5 nm,固定能量窗口Fixed energy window选项,输入100 kcal/mol(419 kJ/mol)。CASTEP模块计算中采用周期边界条件,选取超软赝势和平面波基组展开晶体波函数,交换关联能函数采用广义梯度近似(GGA)中的PW91形式,精度为fine,平面波截止能量设为400 eV。
2. 结果分析与讨论
2.1 重量及脆性分析
不同温度保温30 min的钛氢化反应质量增长率如表1所示。从表1中可看出,400 ℃时质量增长率较低,表明400 ℃下氢化反应不完全,反应温度过低。从450 ℃开始,反应温度越高,质量增长率越大,说明反应温度越高氢化反应越完全。在550 ℃时质量增长率与500 ℃相似,表明氢化反应550 ℃已与500 ℃结果一致,氢化反应已达平衡。综上可以看出较优反应温度为500 ℃。
表 1 不同温度下质量增长率及脆性Table 1. Quality growth rate and brittleness at different temperatures反应温度/℃ 钛质量/g 氢化钛质量/g 质量增加率/% 脆性 400 1.286 1.335 3.8 小 450 2.745 2.858 4.1 小 500 2.225 2.318 4.2 一般 550 2.811 2.929 4.2 大 2.2 形貌分析
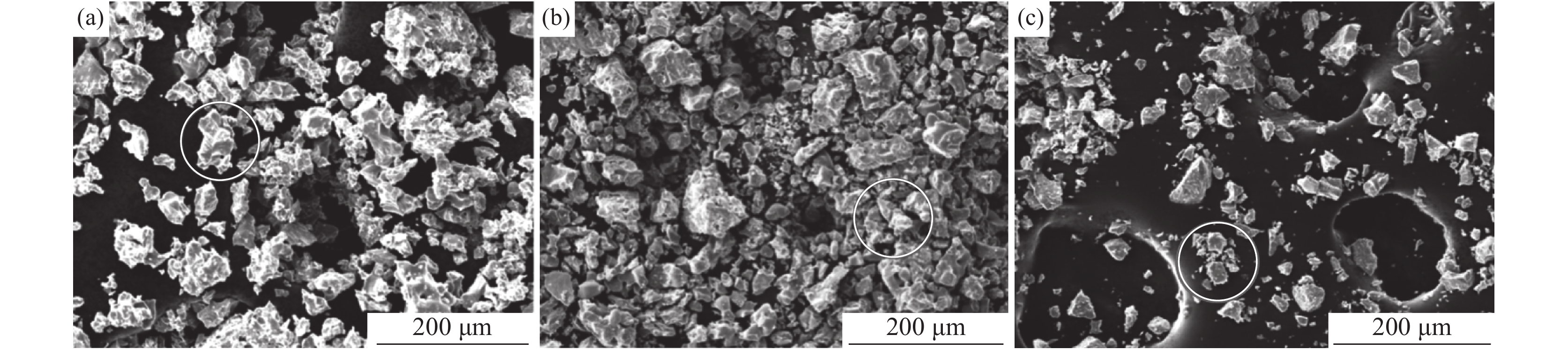
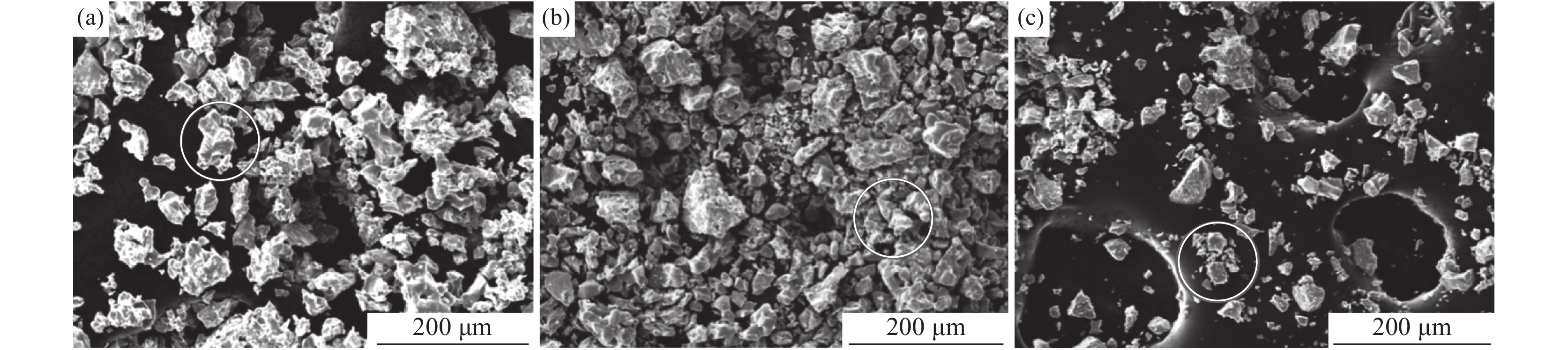
不同温度氢化钛粉SEM形貌如图1所示。由图1可知,氢化钛粉均呈现不规则的多面体结构。不同温度对氢化钛破碎后的颗粒大小有一定影响。400 ℃氢化钛颗粒较大,随温度升高至450 ℃时,大小颗粒均存在。当温度达到500 ℃时,大颗粒的氢化钛已较少。结果表明固体氢化钛的颗粒随反应温度的提高而减小。
2.3 结构分析
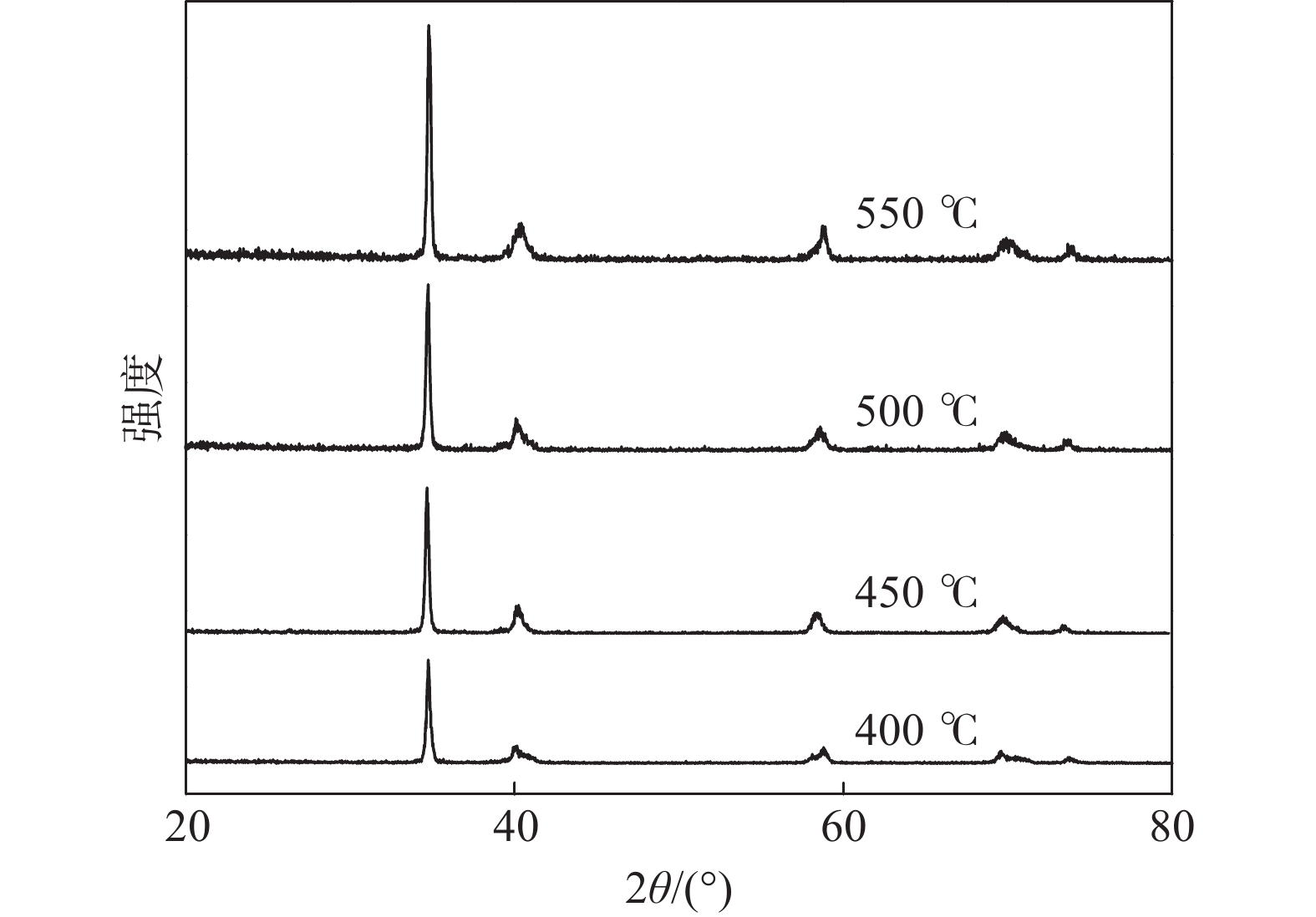
在反应时间30 min,不同反应温度400、450、500、550 ℃下所得产品的XRD衍射谱如图2所示。由图2可知,在400、450、500 ℃和550 ℃下所得产品均为TiH1.924(PDF#25-0982),但500 ℃和550 ℃产品的XRD衍射图谱峰更加尖锐。与TiH1.924标准图谱主峰位对比可知,450 ℃产品第一主峰2θ角为34.720°,500 ℃产品第一主峰2θ角为34.740°,500 ℃产品更接近于标准谱峰,结合表1中不同温度下样品的质量增长率、脆性和节能考虑, 500 ℃较适合氢化钛的制备。
2.4 热力学分析
为分析需要,近似认为钛与氢气在常压、不同温度下反应均生成TiH2,其晶体以TiH2形态存在。即:$ {\text{Ti + }}{{\text{H}}_{\text{2}}}{\text{ = Ti}}{{\text{H}}_{\text{2}}}{\text{ }} $
从文献[9]得到TiH2热力学数据:
△r$ H_{\mathrm{m}}^{\mathrm{\theta}} $(298.15) = −119.66 kJ·mol-1
△r$ {G}_{\mathrm{m}}^{\mathrm{\theta}} $(298.15)= −80.33 kJ·mol-1
Cpm(Ti)= 25.02 J·mol−1·K−1
Cpm(H2)=28.684 J·mol−1·K−1
Cpm(TiH2)= 30.12 J·mol−1·K−1
代入标准反应焓变△r$ {H}_{\rm{m}}^\theta $(T)与温度T的关系,即:
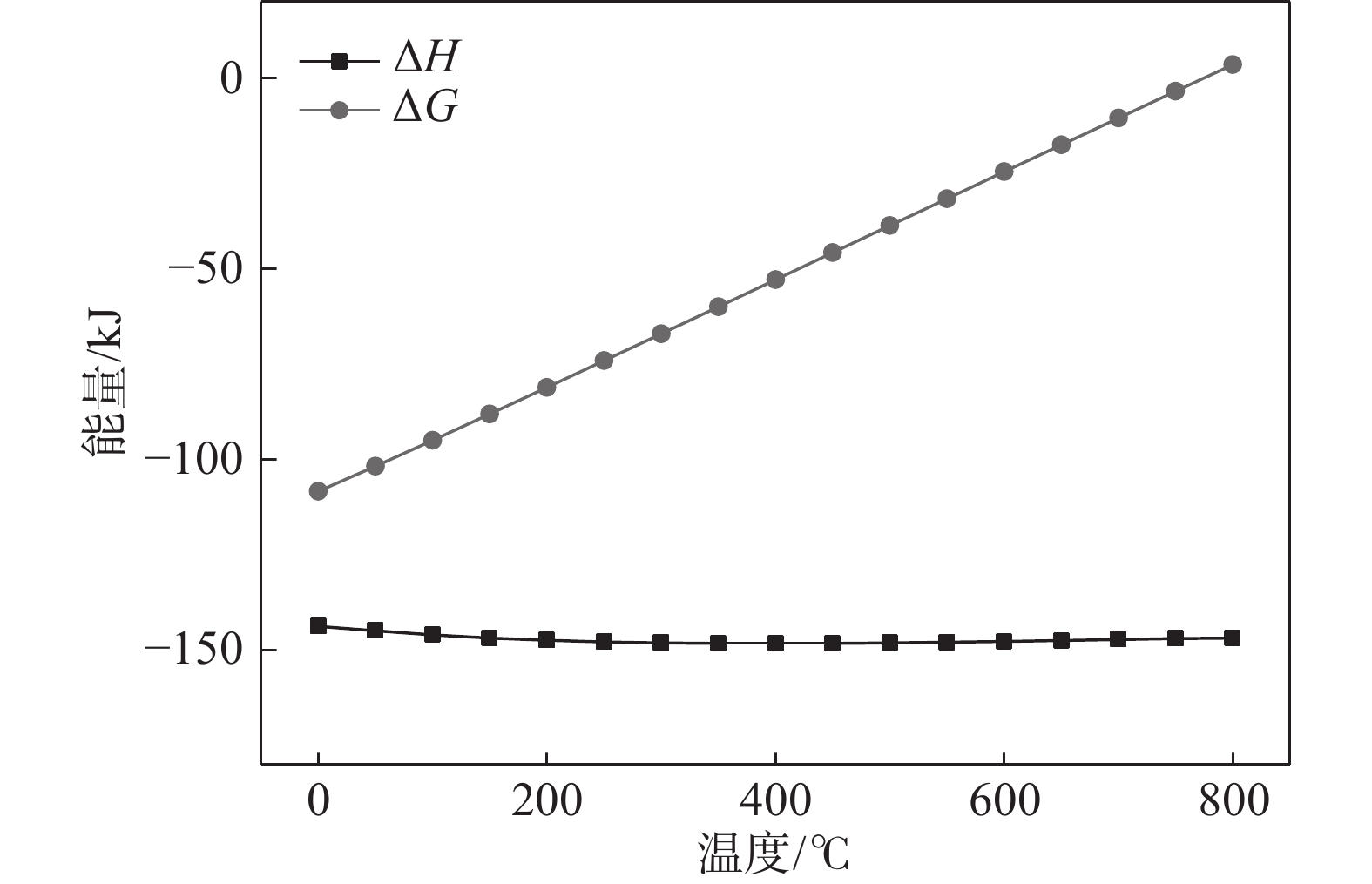
$$ \triangle_{\mathrm{r}} H_{\rm{m}}^\theta(T)=\triangle_{\mathrm{r}} H_{\rm{m}}^\theta(298.15)+\int_{298.15}^{\mathrm{T}} \Delta C_{\mathrm{pm}} {\mathrm{d}} T $$ (3) $$ \Delta_{\mathrm{r}} G_{\rm{m}}^\theta(T)=\Delta_{\mathrm{r}} {G}_{\rm{m}}^\theta(298.15)+\int_{298.15}^{\mathrm{T}} \Delta C_{\mathrm{pm}} {\mathrm{d}} T $$ (4) 可获得标准反应焓变△r$ {H}_{\rm{m}}^\theta $(T)与标准反应吉布期自由能变△r$ {G}_{\rm{m}}^\theta $(T)随温度的变化关系。其结果如图3所示。由图3可知,当温度逐渐升高时,△r$ {H}_{\rm{m}}^\theta $(T)为负值,表明反应为放热反应。但△r$ {G}_{\rm{m}}^\theta $(T)随温度升高,其值逐渐由负变为正值,表明正向反应的驱动力逐渐降低。800 ℃时已为正值,即此时反应已不能自发进行。表明钛的氢化反应应当控制好反应温度,反应温度过高则不能获得需要的产品。
由van’t Hoff方程积分:
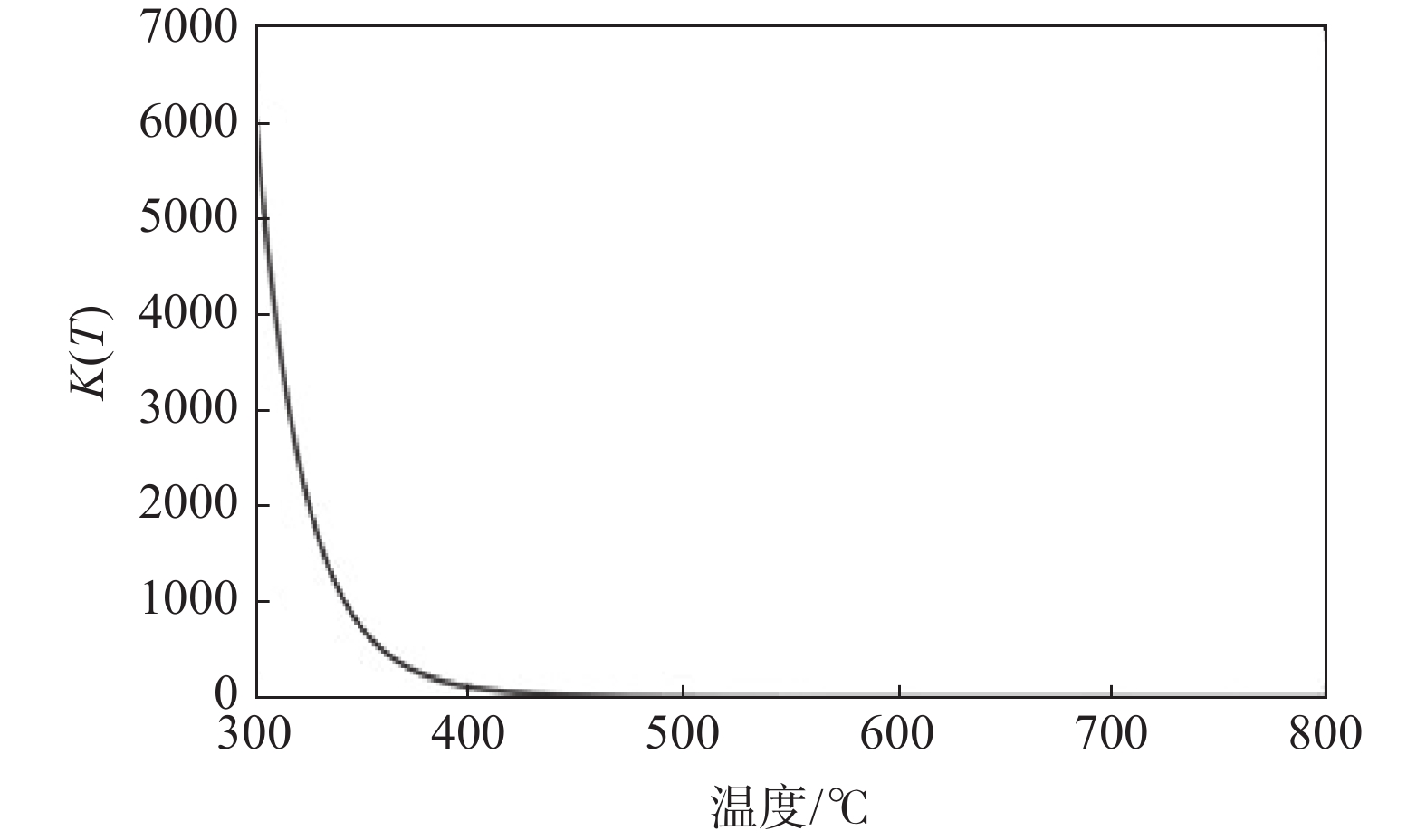
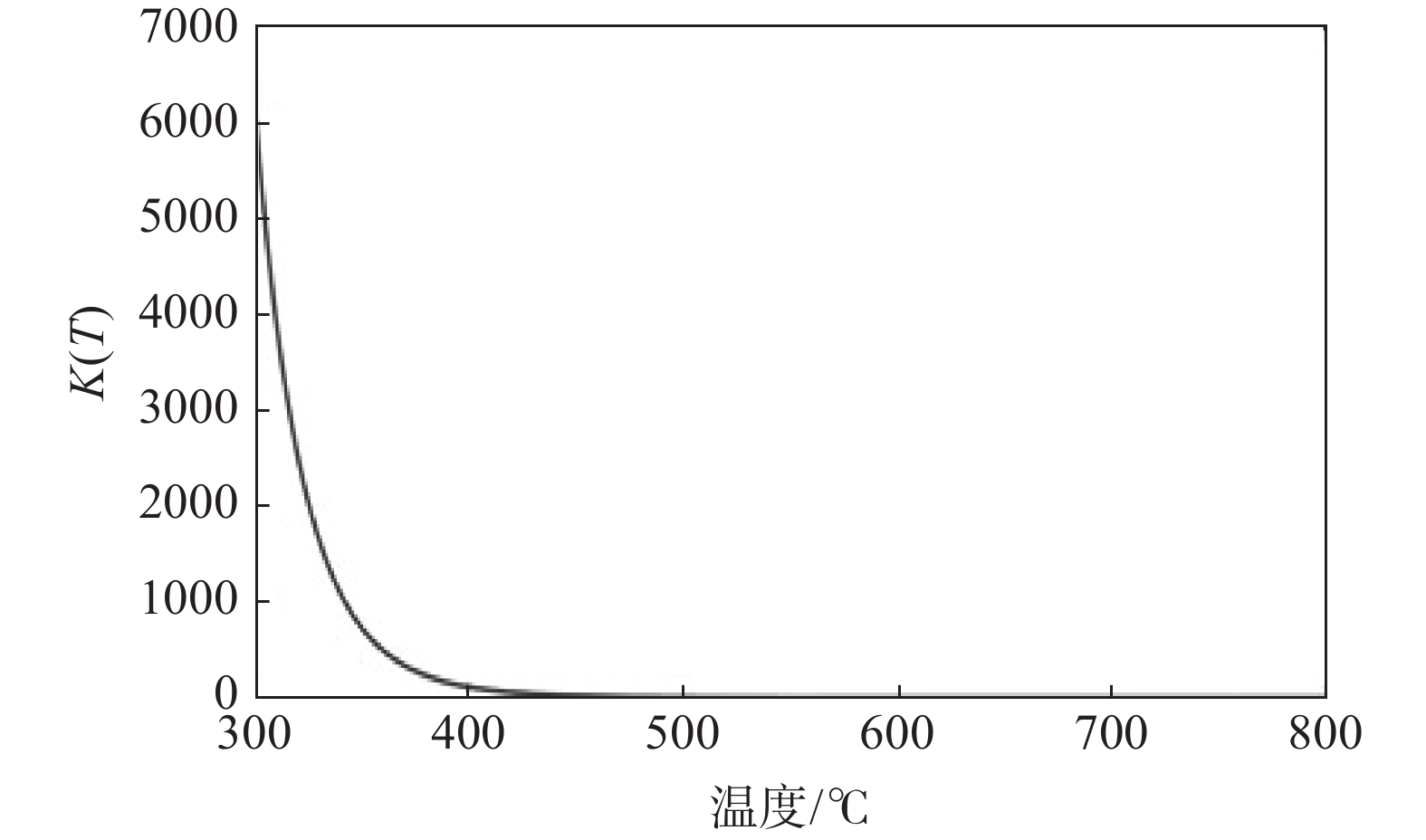
$$ \int_{k(298.15 \;{\mathrm{k}})}^{k(T)} {\mathrm{d}} \ln k=\int_{298.15}^T\left[\Delta_{\mathrm{r}} H_{\mathrm{m}}^\theta(T) / R T^2\right] {\mathrm{d}} T $$ (5) 由此得到反应平衡常数k(T)与T的关系,如图4所示。
由图4的k(T)与T的关系可见,Ti与H2反应的平衡常数随温度T的升高显著下降。这一结果与文献[5]的试验结果较为一致。从图4中可以看出,将反应温度控制在500 ℃左右其平衡常数与反应自由能匹配较好,也与试验结果比较一致。
2.5 第一性原理计算与分析
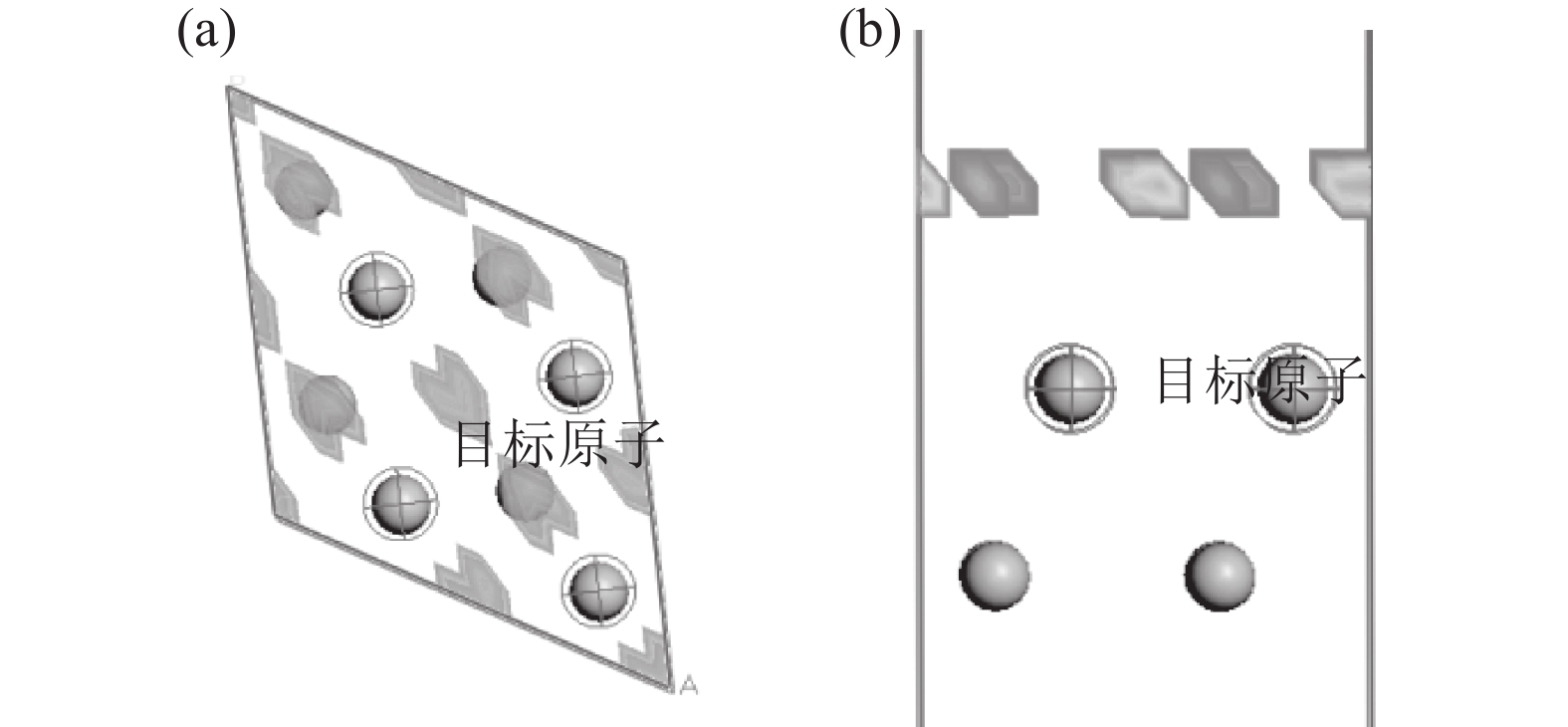
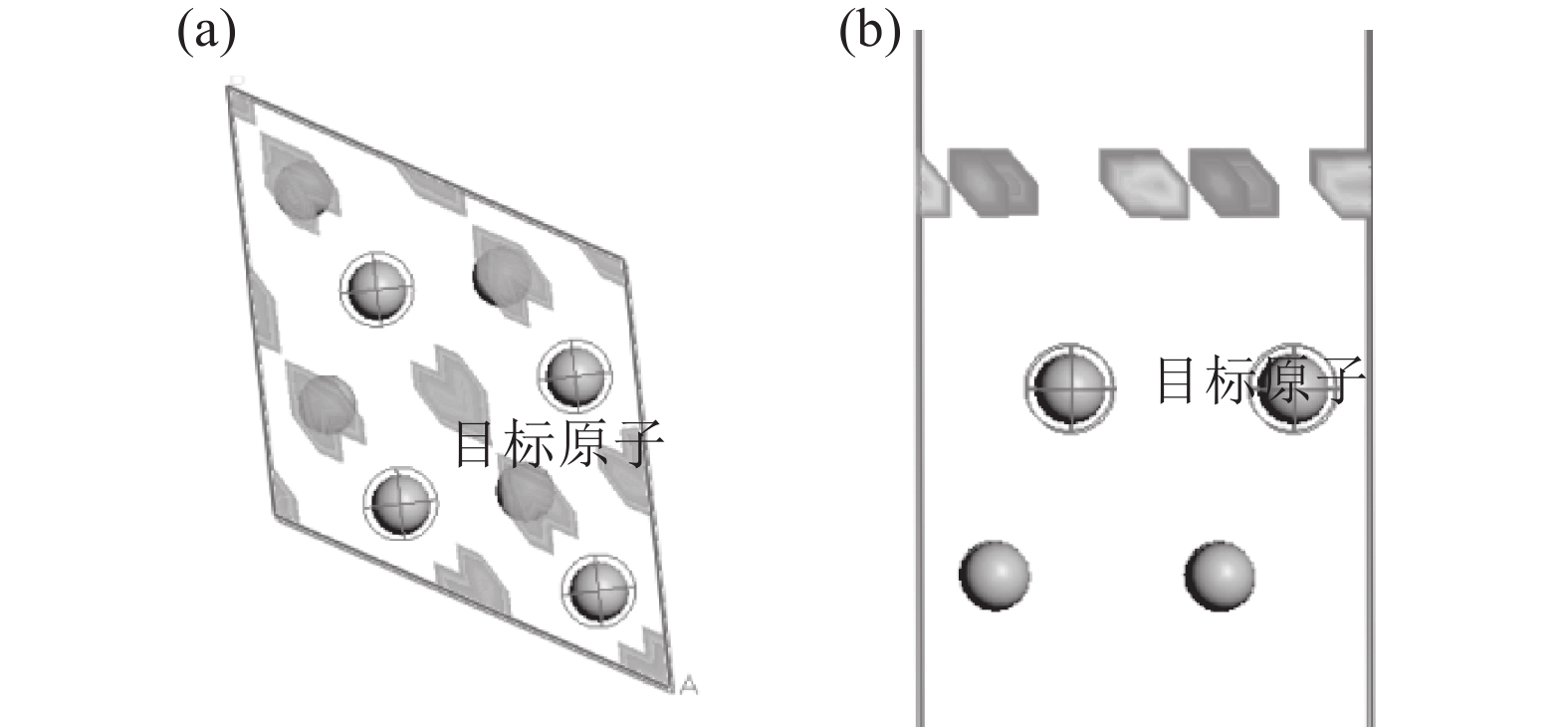
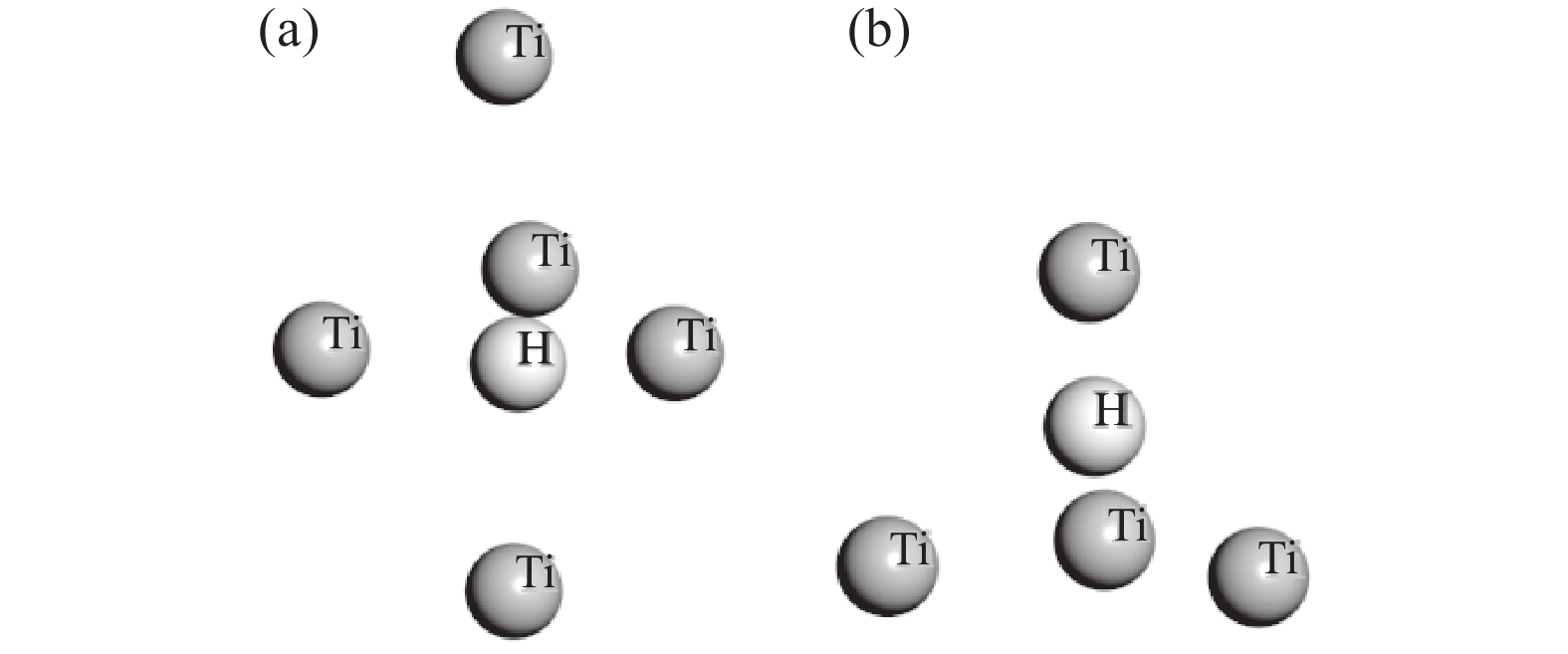
采用Adsorption Locator 模块分析H2分子在Ti表面的吸附位置,得到如图5的较优吸附位。由图5可知,其较优吸附位分为两类:一是上下两层Ti的中心点位;二是单层Ti的三原子中心位。根据其位置关系,采用castep模块计算可以得到H2分子在Ti表面不同位置的吸附能量。其能量最低值在上下两层Ti的中心点位。
当气态H2分子进入金属Ti表面的较优吸附位后,其可能占据的位置有两种,即四面体间隙和八面体间隙。氢原子固溶到金属Ti原子中间的位置如图6所示。氢原子在Ti-H 晶体中占据四面体间隙还是八面体间隙,可以根据从第一个体系到Ti-H 体系的溶解热值来判断,考虑到是放热反应,溶解热值为负值。Ti-H 体系能量越小则绝对值越大,钛氢结构的稳定性越高,相对应的溶解热值就要越小[7-8]。溶解热的计算方程如式(6)所示。
$$ \Delta H=E{_{{\mathrm{t}}_{\mathrm{\left(Ti-H\right)}}}}- \left(E_{{t_{\mathrm{Ti}}}}+\frac{1}{2}E_{{\mathrm{t}}_{\mathrm{H}_2}}\right) $$ (6) 由式(6)可以求得Ti-H体系的溶解热。为此,采用超晶胞方法构建钛与氢摩尔比分别为16∶1、8∶1、4∶1和2∶1的4种Ti-H晶体模型。计算氢的吸附量与能量关系结果列于表2。由表2可知,在Ti-H 体系中,氢原子固溶于四面体的溶解热都大于八面体间隙的溶解热,即八面体间隙的溶解热的绝对值更大。由此可判断氢原子在氢化过程中更倾向于占据八面体间隙。
表 2 氢原子占据α-Ti-H 体系八面体和四面体间隙的溶解热计算结果Table 2. Calculation results of dissolution heat of hydrogen atoms occupying between octahedral and tetrahedral gaps in α-Ti-H system晶体型 位置 Et/eV ΔH(eV·atom-1) α-Ti(2Ti) − 3212.24528 0 16Ti-H 八面体间隙 − 25714.25413 − 0.4082 8Ti-H 八面体间隙 − 12865.36544 − 0.5007 4Ti-H 八面体间隙 − 6425.06412 − 0.57356 2Ti-H 八面体间隙 − 3212.90524 − 0.65996 16Ti-H 四面体间隙 − 3206.24854 − 0.31926 8Ti-H 四面体间隙 − 12841.24458 − 0.36677 4Ti-H 四面体间隙 − 6428.81731 − 0.43658 2Ti-H 四面体间隙 − 3222.60511 − 0.47292 当H原子进入Ti晶格,会使Ti晶格发生膨胀,这是导致金属Ti吸氢后变脆的重要原因。因此,利用第一性原理对模型进行几何优化,可以计算获得吸附氢气后,α-Ti-H体系的体积变化。表3列出了氢原子位于八面体间隙的α-Ti-H 体系的晶体体积的模拟结果。由表3可知,Ti与H原子数量之比从16∶1到2∶1的α-Ti-H体系的体积膨胀率分别从0.17%上升到3.84%。体系的晶格体积增加越严重,金属键越容易断裂的计算结果与随温度升高氢化反应更彻底,其脆性也越大的结果一致[10]。
表 3 氢原子位于八面体间隙的α-Ti-H 体系的体积变化Table 3. Volume change of α Ti-H system of hydrogen atoms located in octahedral gaps% α-Ti(2Ti) 16Ti-H 8Ti-H 4Ti-H 2Ti-H 0 0.17 0.87 1.17 3.84 3. 结论
1)海绵钛和氢气的高温氢化反应在450 ℃时开始进行,反应温度越高,钛的吸氢量越大。产物氢化钛的脆性和质量增加率越大。模拟表明钛的吸氢量变大,Ti与H原子比减小,产物晶格体积膨胀率增加。
2)当反应温度高于500 ℃之后,反应质量增长率变化不大。表明反应已基本达到平衡状态。500 ℃时可获得粒度更细、晶型较优的氢化钛。
3)热力学分析结果表明试验结果与理论温度符合度较高。氢化过程的吸附能量计算表明其较优吸附位位于其中心点位。固溶能量分析表明氢原子更倾向于占据八面体间隙,脆性也越大。
-
表 1 不同温度下质量增长率及脆性
Table 1. Quality growth rate and brittleness at different temperatures
反应温度/℃ 钛质量/g 氢化钛质量/g 质量增加率/% 脆性 400 1.286 1.335 3.8 小 450 2.745 2.858 4.1 小 500 2.225 2.318 4.2 一般 550 2.811 2.929 4.2 大 表 2 氢原子占据α-Ti-H 体系八面体和四面体间隙的溶解热计算结果
Table 2. Calculation results of dissolution heat of hydrogen atoms occupying between octahedral and tetrahedral gaps in α-Ti-H system
晶体型 位置 Et/eV ΔH(eV·atom-1) α-Ti(2Ti) − 3212.24528 0 16Ti-H 八面体间隙 − 25714.25413 − 0.4082 8Ti-H 八面体间隙 − 12865.36544 − 0.5007 4Ti-H 八面体间隙 − 6425.06412 − 0.57356 2Ti-H 八面体间隙 − 3212.90524 − 0.65996 16Ti-H 四面体间隙 − 3206.24854 − 0.31926 8Ti-H 四面体间隙 − 12841.24458 − 0.36677 4Ti-H 四面体间隙 − 6428.81731 − 0.43658 2Ti-H 四面体间隙 − 3222.60511 − 0.47292 表 3 氢原子位于八面体间隙的α-Ti-H 体系的体积变化
Table 3. Volume change of α Ti-H system of hydrogen atoms located in octahedral gaps
% α-Ti(2Ti) 16Ti-H 8Ti-H 4Ti-H 2Ti-H 0 0.17 0.87 1.17 3.84 -
[1] Feng Yingfang, Zhang Zhen. Production and application of titanium powder[J]. Titanium Industry Progress, 2000(6):6-9. (冯颖芳, 张震. 钛粉的生产及应用[J]. 钛工业进展, 2000(6):6-9. doi: 10.3969/j.issn.1009-9964.2000.06.002Feng Yingfang, Zhang Zhen. Production and application of titanium powder[J]. Titanium Industry Progress, 2000(6): 6-9. doi: 10.3969/j.issn.1009-9964.2000.06.002 [2] Hong Yan, Qu Tao, Shen Huasen, et al. Titanium production through hydrogenation and dehydrogenation process[J]. Chinese Journal of Rare Metals, 2007(3):311-315. (洪艳, 曲涛, 沈化森, 等. 氢化脱氢法制备钛粉工艺研究[J]. 稀有金属, 2007(3):311-315. doi: 10.3969/j.issn.0258-7076.2007.03.007Hong Yan, Qu Tao, Shen Huasen, et al. Titanium production through hydrogenation and dehydrogenation process[J]. Chinese Journal of Rare Metals, 2007(3): 311-315. doi: 10.3969/j.issn.0258-7076.2007.03.007 [3] Huang Guangming, Lei Ting, Fang Shuming, et al. Research progress of preparation powders by titanium hydrogenation[J]. Titanium Industry Progress, 2010(6):6-9. (黄光明, 雷霆, 方树铭, 等. 氢化脱氢制备钛粉的研究进展[J]. 钛工业进展, 2010(6):6-9. doi: 10.3969/j.issn.1009-9964.2010.05.002Huang Guangming, Lei Ting, Fang Shuming, et al. Research progress of preparation powders by titanium hydrogenation[J]. Titanium Industry Progress, 2010(6): 6-9. doi: 10.3969/j.issn.1009-9964.2010.05.002 [4] Liu Jie, Shang Qingliang, Zhang Wei, et al. Research progress in preparation of titanium and titanium alloy materials by titanium hydride powder[J]. Materials Review, 2013,13:99-102. (刘捷, 尚青亮, 张炜, 等. 氢化钛粉制备钛及钛合金材料研究进展[J]. 材料导报, 2013,13:99-102. doi: 10.3969/j.issn.1005-023X.2013.01.019Liu Jie, Shang Qingliang, Zhang Wei, et al. Research progress in preparation of titanium and titanium alloy materials by titanium hydride powder[J]. Materials Review, 2013, 13: 99-102. doi: 10.3969/j.issn.1005-023X.2013.01.019 [5] Chen Song, Xiao Sufen, Wang Chunming, et al. Hydrogen dehydrogenation kinetics of titanium sponge from Panzhihua[J]. Functional Materials, 2014,45(11):11123-11125, 11131. (陈松, 肖素芬, 王春明, 等. 攀枝花产海绵钛氢化脱氢动力学[J]. 功能材料, 2014,45(11):11123-11125, 11131. doi: 10.3969/j.issn.1001-9731.2014.11.026Chen Song, Xiao Sufen, Wang Chunming, et al. Hydrogen dehydrogenation kinetics of titanium sponge from Panzhihua[J]. Functional Materials, 2014, 45(11): 11123-11125, 11131. doi: 10.3969/j.issn.1001-9731.2014.11.026 [6] Han Xiuli, Wang Qing, Sun Dongli, et al. First-principle calculation of crystal structure and energies of Ti-H system[J]. The Chinese Journal of Nonferrous Metals, 2008(3):523-528. (韩秀丽, 王清, 孙东立, 等. 钛-氢体系晶体结构和能量的第一原理计算[J]. 中国有色金属学报, 2008(3):523-528. doi: 10.3321/j.issn:1004-0609.2008.03.024Han Xiuli, Wang Qing, Sun Dongli, et al. First-principle calculation of crystal structure and energies of Ti-H system[J]. The Chinese Journal of Nonferrous Metals, 2008(3): 523-528. doi: 10.3321/j.issn:1004-0609.2008.03.024 [7] Lu Jinlian, Cao Juexian. A first-principles study of capacity and mechanism of a single titanium atom storing hydrogen[J]. Acta Physica Sinica, 2012,45(14):497-502. (卢金炼, 曹觉先. 单个钛原子储氢能力和储氢机制的第一性原理研究[J]. 物理学报, 2012,45(14):497-502. doi: 10.7498/aps.61.148801Lu Jinlian, Cao Juexian. A first-principles study of capacity and mechanism of a single titanium atom storing hydrogen[J]. Acta Physica Sinica, 2012, 45(14): 497-502. doi: 10.7498/aps.61.148801 [8] Zhang Jian, Zhou Dianwu, Liu Jinshui. Study on hydrogen atom adsorption and diffusion properties on Mg(0001) surface[J]. Scientia Sinica(Technologica), 2009,39(8):1440-1447. (张健, 周惦武, 刘金水. H原子在Mg(0001)表面的吸附与扩散性能研究[J]. 中国科学: 技术科学, 2009,39(8):1440-1447.Zhang Jian, Zhou Dianwu, Liu Jinshui. Study on hydrogen atom adsorption and diffusion properties on Mg(0001) surface[J]. Scientia Sinica(Technologica), 2009, 39(8): 1440-1447. [9] Li Guangming, Gan Lihua, Chen Longwu, et al. The formation and decomposition of titanium hydride[J]. Chinese Journal of Applied Chemistry, 1998,01:77-79. (李光明, 甘礼华, 陈龙武,等. 氢化钛的制备及其分解[J]. 应用化学, 1998,01:77-79.Li Guangming, Gan Lihua, Chen Longwu, et al. The formation and decomposition of titanium hydride[J]. Chinese Journal of Applied Chemistry, 1998, 01: 77-79. [10] Wei Shengquan, Zheng Yubin, Wang Yujie, et al. First-principles calculation of geometry structures and energies of α-Ti-H system[J]. Journal of Netshape Forming Engineering, 2015(1):27-30. (魏圣泉, 郑玉斌, 王玉洁, 等. 置氢α钛几何与能量的第一性原理研究[J]. 精密成形工程, 2015(1):27-30. doi: 10.3969/j.issn.1674-6457.2015.01.005Wei Shengquan, Zheng Yubin, Wang Yujie, et al. First-principles calculation of geometry structures and energies of α-Ti-H system[J]. Journal of Netshape Forming Engineering, 2015(1): 27-30. doi: 10.3969/j.issn.1674-6457.2015.01.005 -





 下载:
下载:






 下载:
下载: