Current development status of hydrogen reduction technology with the CH4-H2 system
-
摘要: 在“双碳”目标与“双高”限制的双重挑战下,火法冶金领域正面临能耗与碳排放的双重压力,对清洁能源的需求愈发迫切。氢能,作为一种可再生清洁能源,为火法冶金带来了节能低碳、绿色转型的新曙光。基于CH4-H2体系的氢还原技术,凭借其优良的还原能力和低碳无污染的特性,已成为冶金领域研究的热点。阐述了CH4-H2体系的还原热力学与动力学原理,以及国内外相关技术及研究进展,总结了该技术在还原金属矿物(铁、钛、镍、锌、钴、铬、锰)方面的研究成果与发展方向,并针对尚未解决的问题进行了系统分析。基于CH4-H2体系的氢还原技术有巨大的应用潜力,这些探讨将有助于推动该技术的进一步发展。Abstract: Under the dual challenges posed by the “dual carbon” goals and “double high” restrictions, the field of pyrometallurgy is experiencing significant pressures related to energy consumption and carbon emissions, thereby heightening the urgent need for clean energy. Hydrogen energy, as a renewable clean energy source, offers a new dawn for pyrometallurgy in terms of energy-saving, low-carbon, and green transformation. Hydrogen reduction technology based on the CH4-H2 system, with its excellent reduction capability and low-carbon, pollution-free characteristics, has emerged as a research hotspot in the metallurgical field. This paper elucidates the reduction thermodynamics and kinetics principles of the CH4-H2 system, systematically reviews the relevant domestic and international technologies and research progress, summarizes the research achievements and development directions of this technology in the reduction of metallic minerals (iron, titanium, nickel, zinc, cobalt, chromium, and manganese), and conducts a systematic analysis of the unresolved issues. The hydrogen reduction technology based on the CH4-H2 system holds immense application potential, and these discussions will contribute to advancing the further development of this technology.
-
Key words:
- metallic mineral /
- pyrometallurgy /
- hydrogen reduction technology /
- CH4-H2 system
-
0. 引言
国际能源署(IEA)《2023年碳排放报告》显示,全球能源相关CO2排放量创新高,达374亿t,中国占比超33%。作为工业大国,中国钢铁与有色金属行业的碳排放总量约占全国碳排放总量的19.7%[1]。火法冶金是目前国内外冶炼钢铁和大部分有色金属的主要方式,具有工艺简单、处理量大、适用性强等优点。火法冶金过程除电力消耗外,大量的煤炭资源被直接用于还原金属氧化物和燃烧提供热量,如铁、钛、钒等元素矿物及含有价金属元素固体废弃物的提取冶金过程均消耗煤炭资源,是典型的高能耗、高碳排放“碳冶金”过程,会不可避免地产生大量的CO2排放。
在全面“脱碳”背景下,各行业都围绕绿色发展主题,积极履行减排责任,制定减排目标。我国正处于经济建设的上升期,钢铁、有色金属等高能耗、高排放的支柱性产业面临巨大的碳减排压力。因此,工业生产能源转型势在必行,而绿色可再生能源的大规模应用是实现节能减排目标的重要手段之一。氢能作为二次可再生清洁能源,来源广,还原性强,被视为21世纪最具发展潜力的绿色可再生能源,已被许多国家纳入国家能源战略部署[2]。目前,“以氢代碳”是国内外金属矿石冶炼行业从源头减少碳排放的重点发展方向,而基于氢能的氢还原技术也成为火法冶金领域推进节能低碳、绿色发展的有效途径之一[3]。
氢能泛指以“氢”为主要载体转化形成的二次能源,除H2本身以外,CH4、NH3等也是典型的氢能资源。其中CH4气体来源十分广泛,是页岩气和焦炉煤气的主要成分,也是一种高效的还原剂和碳化剂,因此受到相关研究者的广泛关注[4]。近些年来,研究者们基于CH4-H2体系,对多种金属氧化物的还原反应开展了研究,结果证实,相比于常规碳热还原,基于CH4-H2体系的氢还原技术能够在更低温度下还原金属氧化物。笔者分析了CH4-H2体系的还原热力学与动力学原理,对国内外基于CH4-H2体系的氢还原技术及研究进展进行了系统综述,阐述了CH4-H2体系还原金属矿物的研究和发展方向,并针对此技术目前在工业化应用中存在的问题进行了分析,有助于促进氢还原技术的发展。
1. CH4还原金属氧化物的热力学和动力学
基于CH4-H2体系的氢还原技术,其核心并非CH4直接还原金属氧化物。实际上,CH4在高温下裂解生成的H2和高活性C才是还原和碳化的关键。此过程中,CH4首先在金属氧化物表面吸附并裂解,其基本反应是CH4分解为H2和C,如式(1)~(4)所示。
$$ \mathrm{CH}_4=\mathrm{CH}_3+[\mathrm{H}] $$ (1) $$ \mathrm{CH}_3=\mathrm{CH}_2+[\mathrm{H}] $$ (2) $$ \mathrm{CH}_2=\mathrm{CH}+[\mathrm{H}] $$ (3) $$ \mathrm{CH}=\mathrm{C}+[\mathrm{H}] $$ (4) 式中[H]表示解离的氢原子。
金属氧化物MeO与CH4的整体还原反应如式(5)所示。根据式(4),体系中存在的单质C在高温下会与CO2发生如式(6)所示的Boudouard反应[5]。水蒸气也可以在高温下与C发生如式(7)所示的水煤气反应。沈峰满等[6−9]基于物质衡算及化学热力学平衡原理,构建了“H-C-O体系质量及化学平衡衡算图”,并对涉及CH4-H2-CO-H2O-CO2体系的析碳共性问题进行了研究。其结果表明,体系中H2/CO的比例较高时,甲烷的分解反应成为主流,CO的析碳反应(式(6)的逆向反应)会受到抑制,式(8)的水煤气变换反应倾向于逆向进行。此外,由于反应(6)和(7)均为吸热反应,反应(8)为放热反应,温度的升高也会促进体系中H2O和CO2不断向H2和CO转变。因此,在CH4通入过量的情况下,金属氧化物MeO与CH4在高温下的整体反应如式(9)所示。
$$ 4 \mathrm{MeO}(\mathrm{s})+\mathrm{CH}_4(\mathrm{g}) \rightarrow 4 \mathrm{Me}(\mathrm{s})+\mathrm{CO}_2(\mathrm{g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) $$ (5) $$ \mathrm{C}(\mathrm{s})+\mathrm{CO}_2(\mathrm{g}) \rightarrow 2 \mathrm{CO}(\mathrm{g}) $$ (6) $$ \mathrm{C}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightarrow \mathrm{CO}(\mathrm{g})+\mathrm{H}_2(\mathrm{g}) $$ (7) $$ \mathrm{CO}(\mathrm{g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightarrow \mathrm{CO}_2(\mathrm{g})+\mathrm{H}_2(\mathrm{g}) $$ (8) $$ 4 \mathrm{MeO}(\mathrm{s})+\mathrm{CH}_4(\mathrm{g}) \rightarrow 4 \mathrm{Me}(\mathrm{s})+\mathrm{CO}(\mathrm{g})+2 \mathrm{H}_2(\mathrm{g}) $$ (9) CH4在
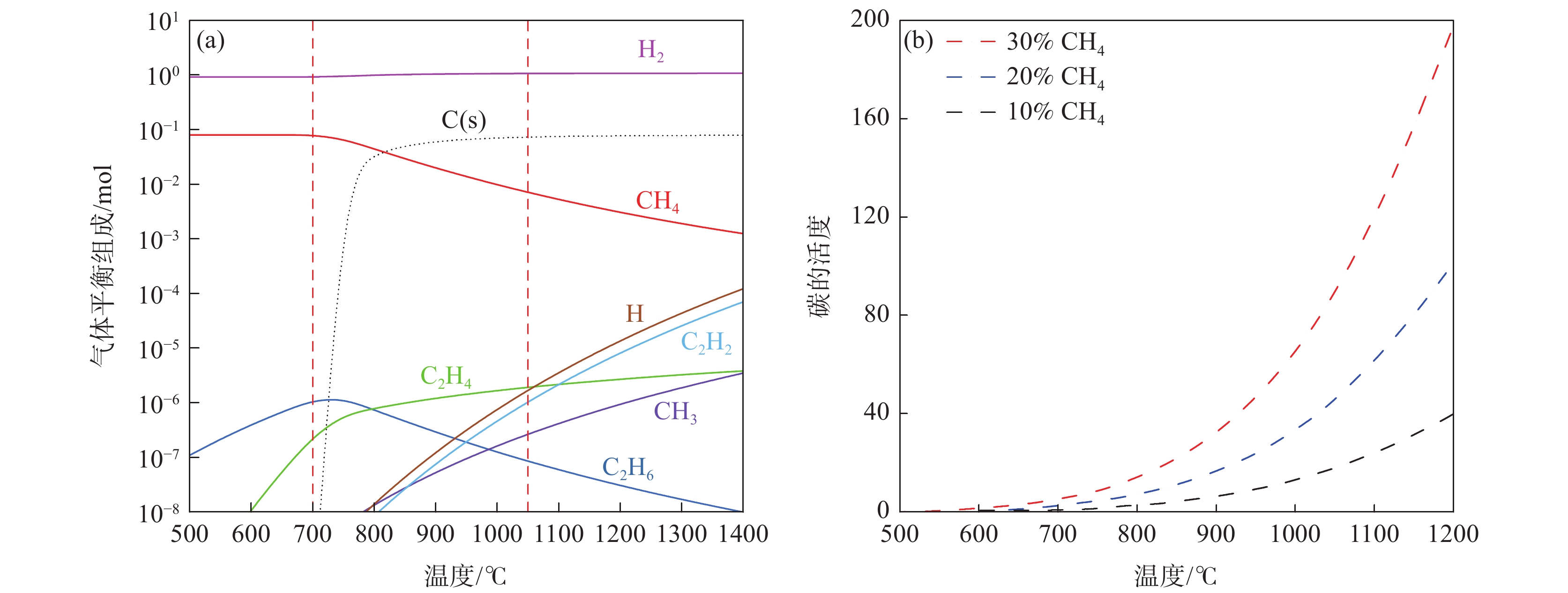
1000 ℃以上的迅速裂解会导致样品表面积碳,影响还原反应的进行。因此,研究者们一般在较低温度下采用纯CH4或CH4-Ar混合气体作为还原剂,高温下(1000 ℃以上)采用CH4-H2/CH4-H2-Ar混合气体作为还原剂,H2的存在既能充当还原剂,也能抑制CH4的裂解速率。张润等[10]利用Factsage软件计算了1 mol CH4-H2混合气体(CH4∶H2=8∶92)在不同温度下的平衡物相。结果表明(图1(a)),混合气体中的CH4在700 ℃以下能够稳定存在。随着温度的逐渐升高,CH4会大量裂解生成H2和C,但是即便在1050 ℃下也有约10%的CH4稳定存在,气体产物中也残留较多的C2H2和C2H4等不完全裂解产物,这表明H2在高温下能有效抑制CH4裂解。CH4裂解产生的碳是纳米级的,其热力学活度大于1(以石墨为基准)。Halli等[11]对CH4-H2体系中碳的热力学活度进行了计算,结果如图1(b)所示。随着温度的增加和体系中CH4浓度的增加,碳的热力学活度明显增加,碳浓度也随CH4裂解速率的增加而增加。CH4-H2体系中碳的高活性和高浓度使得多数情况下金属氧化物的最终产物不是纯金属相,而是相对稳定的碳化物。
理论上,基于CH4-H2体系的氢还原技术对采用碳热还原冶炼的金属矿石均有潜在应用前景,但实际操作中需重视动力学限制因素。动力学模型构建对于解释试验数据、设计和放大反应器具有重要意义,有助于确定操作参数,更好地控制还原过程。基于CH4-H2体系的氢还原金属氧化物是典型的气-固反应,甲烷还原金属氧化物的过程可分为九个不同的步骤:①甲烷的外部扩散;②甲烷的内部扩散;③CH4的物理吸附;④CH4的化学吸附;⑤界面处的化学反应;⑥气体产物(CO, H2)的化学解吸;⑦气态产物(CO, H2)的物理解吸;⑧气体产物(CO, H2)的内部扩散;⑨气体产物(CO, H2)的外部扩散。
目前文献中已有许多模型用于描述CH4还原金属氧化物的动力学过程,这些模型包括非多孔固体的未反应收缩核模型、多孔固体的体积反应模型、颗粒模型、修正颗粒模型和随机孔隙模型等。
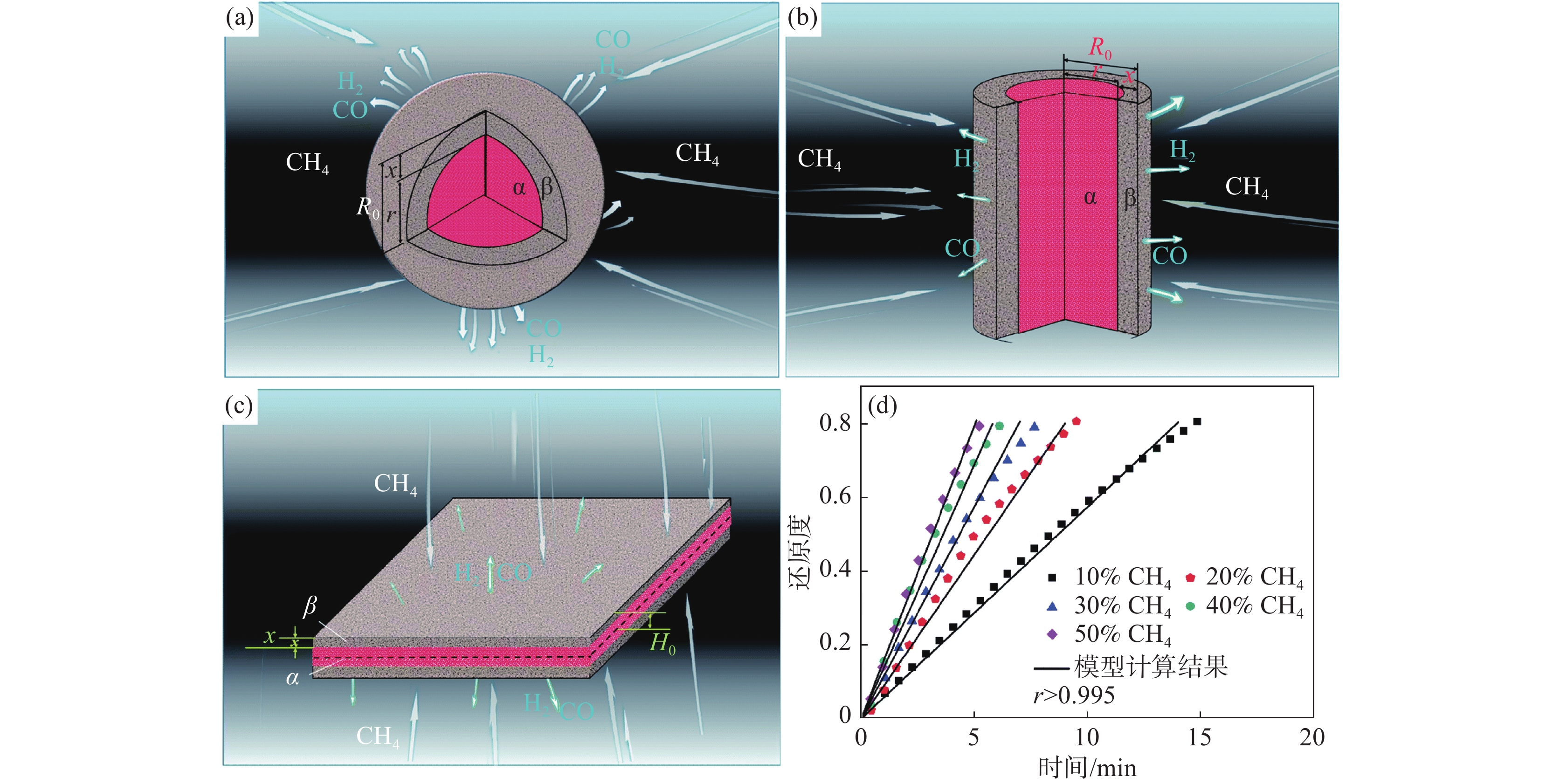
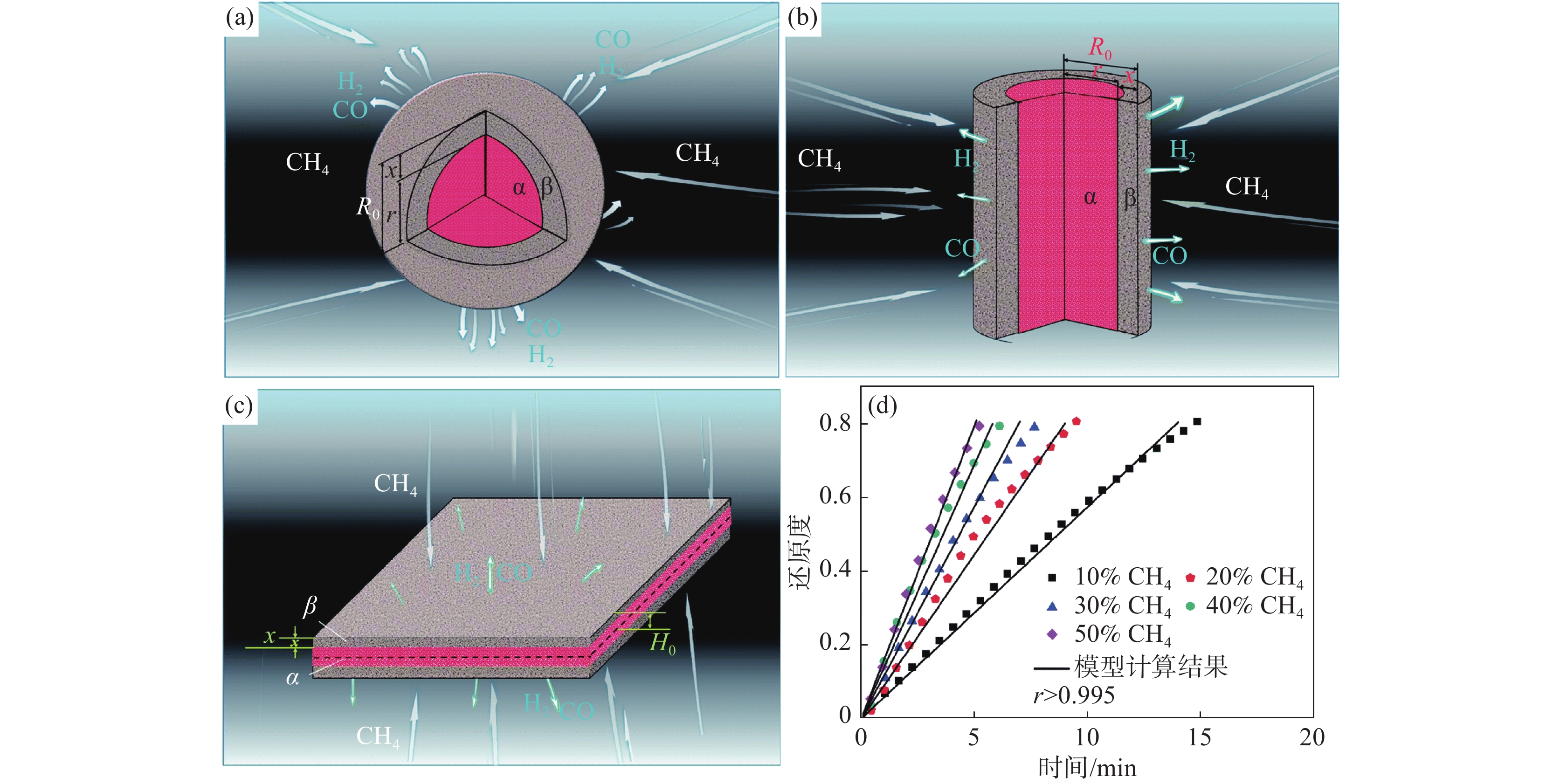
吕泽鹏等[12]构建了一种多界面反应模型来模拟金属氧化物与CH4的反应,如图2所示。该模型包含了三种形状(球形、圆柱形和层状)的颗粒模型,同时考虑了在化学反应控制条件下,CH4裂解速率对反应速率的影响。模型预测结果与Rashidi等[13]的NiO还原试验数据匹配度良好,说明所建立的模型可以很好地描述单一金属氧化物与CH4的反应过程。然而,此模型为了降低复杂度忽略了传热等参数的影响,因此对于不规则复杂氧化物颗粒的还原过程,该模型的预测准确率还有待商榷。
CH4裂解产生的高活性碳在热力学上具有极高的活度,这一活度远超石墨,并且随着温度的升高和CH4浓度的增加,其活度会显著增强。因此,相较于传统的碳热还原过程,CH4在热力学上展现出了在更低温度下还原金属氧化物的优势。此外,这种高活性碳还更容易与金属单质反应,形成金属碳化物,这进一步拓宽了CH4在金属氧化物还原中的应用范围。在气-固反应过程中,CH4在样品表面的吸附与解离起到了至关重要的作用。这一过程确保了裂解产生的H2和高活性碳能够与样品充分接触,从而提高了反应的效率和速率。与固-固反应的传统碳热还原过程相比,CH4还原金属氧化物的反应活化能通常更低,这意味着反应更容易进行,且还原速率更快。
2. 基于CH4-H2体系的铁氧化物还原研究
铁氧化物在CH4-H2气氛中可还原为铁,伴生氢气、一氧化碳等气体产物,且特定条件下会发生渗碳反应生成碳化铁(Fe3C)。倪红卫等[14]研究发现,600 ℃常压下,赤铁矿粉制备碳化铁的热力学最优X($ \mathit{\mathrm{\mathit{P}}}_{\mathrm{H}_2}/\mathrm{\mathit{P}}_{\mathrm{CH}_4} $)值为1.7(氢气63%),但试验显示50% CH4+50% H2的气体配比更理想,且N2含量需<5%,碳化铁在还原度约45%时才开始生成。马江华等[15]发现疏松多孔结构的铁矿石还原速率较快,且生成的还原铁结构同样疏松,利于后续的碳化反应。李光强等[16−17]在650~800 ℃温度范围内,通过H2还原后采用CH4碳化处理高磷鲕状赤铁矿,经球磨磁选后可制得平均粒径约2.5 μm的碳化铁。他们也发现V2O5和Al2O3作为促进剂能加速碳化铁生成,MnO和Cr2O3影响较小,而Na2SO4和TiO2则会抑制碳化铁生成。
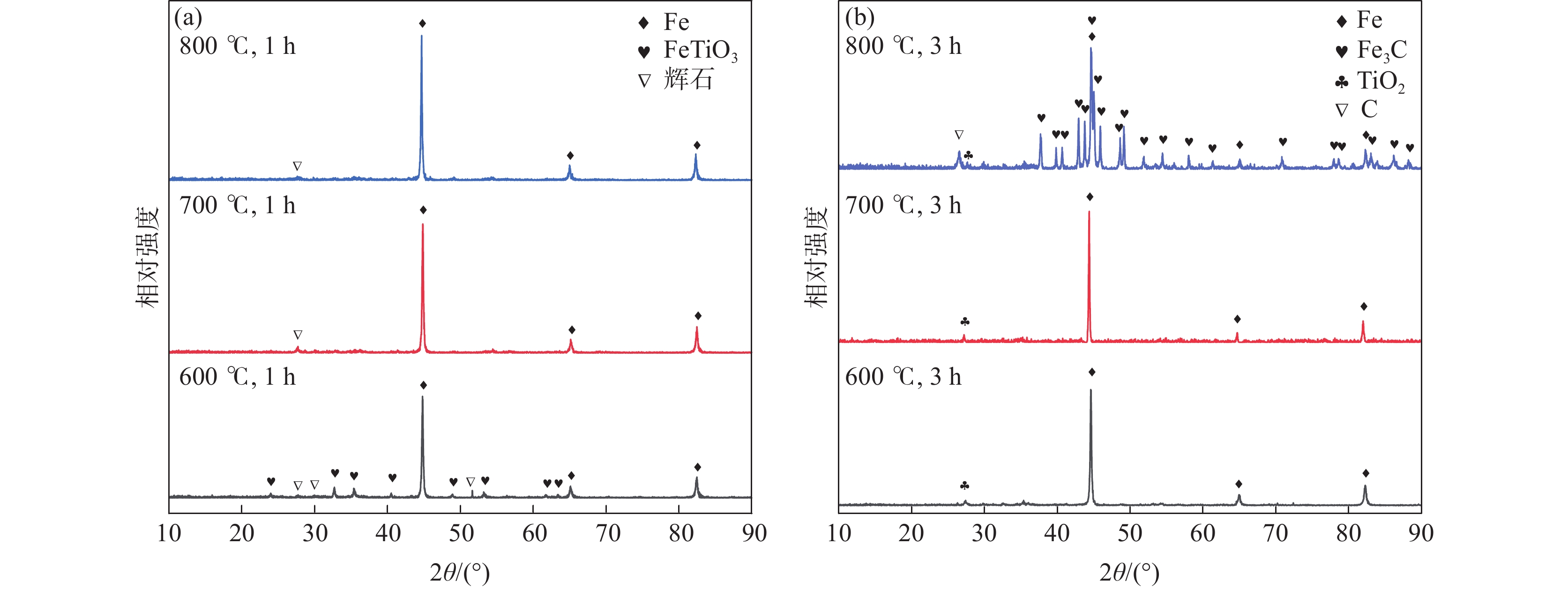
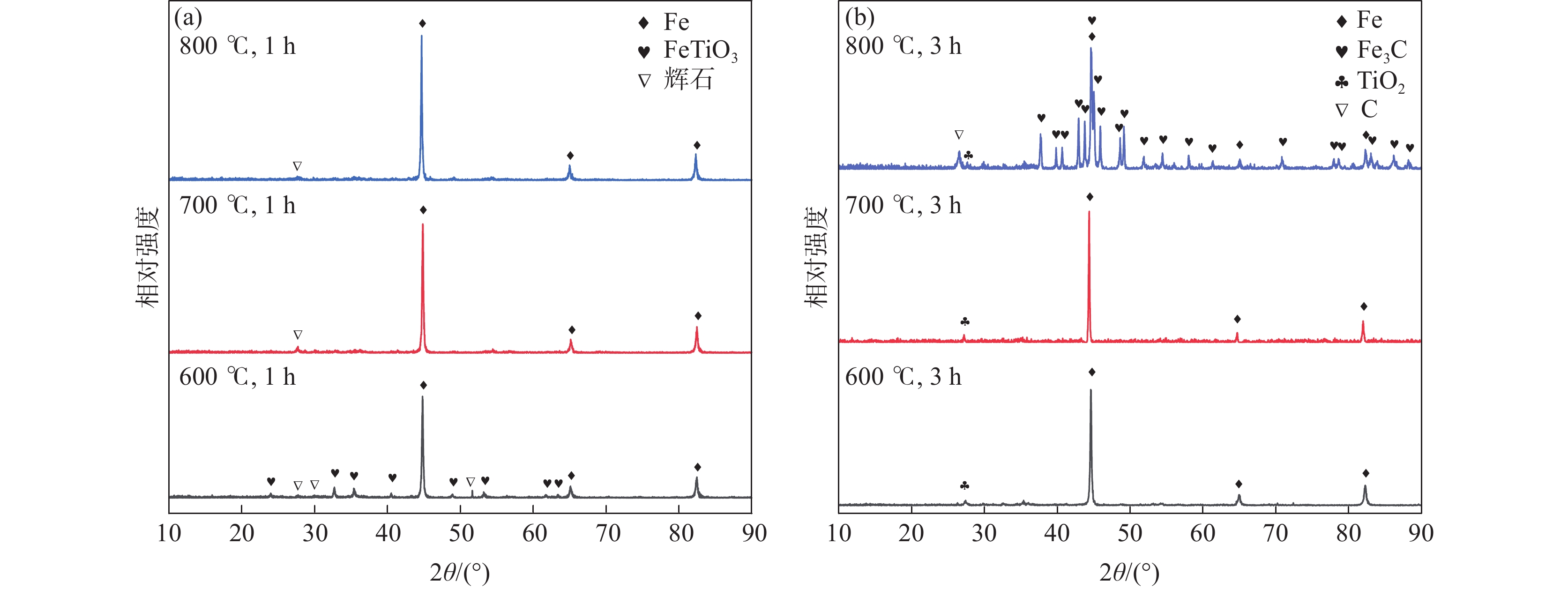
张润等人[18]探讨了CH4-H2混合气体直接还原攀枝花铁精矿制备Fe3C的可行性。研究指出,FeTiO3完全转化为Fe3C的理论温度需超过709 ℃,而试验中(图3)铁精矿在800 ℃以上能被部分碳化为Fe3C,产物中仍含有大量金属铁。提高温度或延长反应时间均未能实现铁的完全碳化,综合分析认为其可能与TiO2的存在或者高温下Fe3C的分解有关。
Ghosh等[19]发现,CH4还原致密氧化铁球团的反应活化能分为两阶段:875~950 ℃时为220.2 kJ/mol,950~
1025 ℃时为105.7 kJ/mol。反应的控制步骤可能是CH4的分解速率,活化能的降低与体系中随温度升高而增加的氢气浓度有关。Monazam等[20]对赤铁矿在流动CH4气氛中的还原行为进行了热重分析(TGA),发现700~825 ℃下赤铁矿的还原动力学涉及两个相互竞争的反应过程:①一级不可逆反应和②Avrami指数为n=2的成核-生长过程。两个反应的活化能分别为(34.4±0.5) kJ/mol和(39.3±1.6) kJ/mol,反应速率随温度和入口气体中CH4浓度的增加而增加。在CH4-H2-(N2)体系还原铁氧化物的研究中,研究者们通过优化混合气体配比、矿粉粒度和添加物种类与用量等试验参数,以提高铁氧化物的还原效率和碳化产物的质量,并详细探讨了还原动力学。然而,因试验条件差异等原因,反应活化能计算结果不一,动力学机制需要进一步深入剖析。此外,现有研究主要集中于纯铁氧化物和高品位铁矿石,未来应在既有理论与试验方法的基础上,转向探索低品位复杂铁矿的利用。
3. 基于CH4-H2体系的钛氧化物还原研究
CH4近些年来也被相关研究者用于钛氧化物的还原。Zhang等人[21−22]利用CH4-H2-Ar混合气体对多种含钛原料进行了还原碳化试验,发现TiO2在
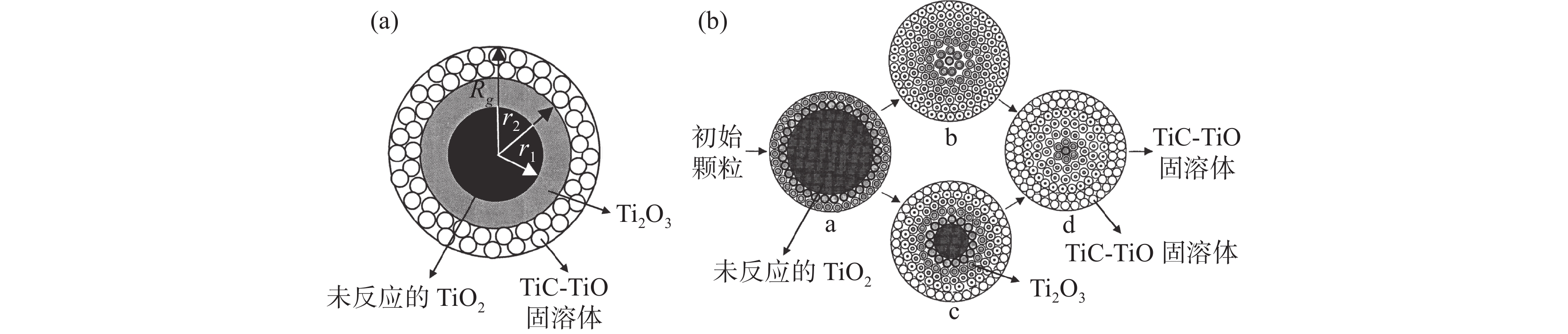
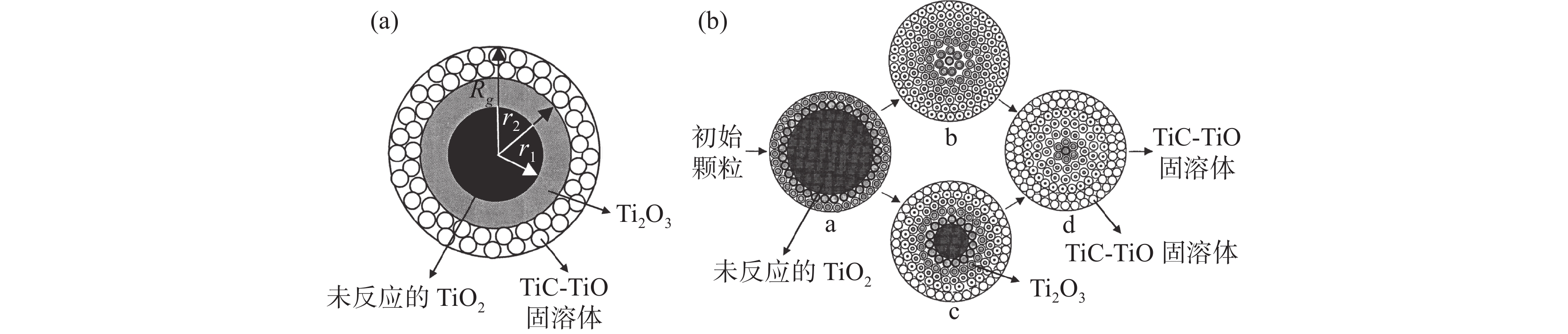
1100 ℃便能被还原为Ti(C, O)(TiC和TiO的固溶体),CH4的最佳比例为8%,H2的比例应保持在35%以上。作者采用双界面收缩核模型和裂核模型(图4)对TiO2的两个还原反应步骤(1)TiO2→Ti2O3和(2)Ti2O3→Ti(C,O)进行了动力学建模[23]。结果显示,在1200 ~1500 ℃下,两步骤均由界面化学反应控制,裂核模型与试验数据的吻合性相对更好。党杰等[24−25]利用CH4-H2-Ar的混合气体对高钛渣和钛铁矿进行了还原试验研究。结果表明,高钛渣的最佳还原温度为
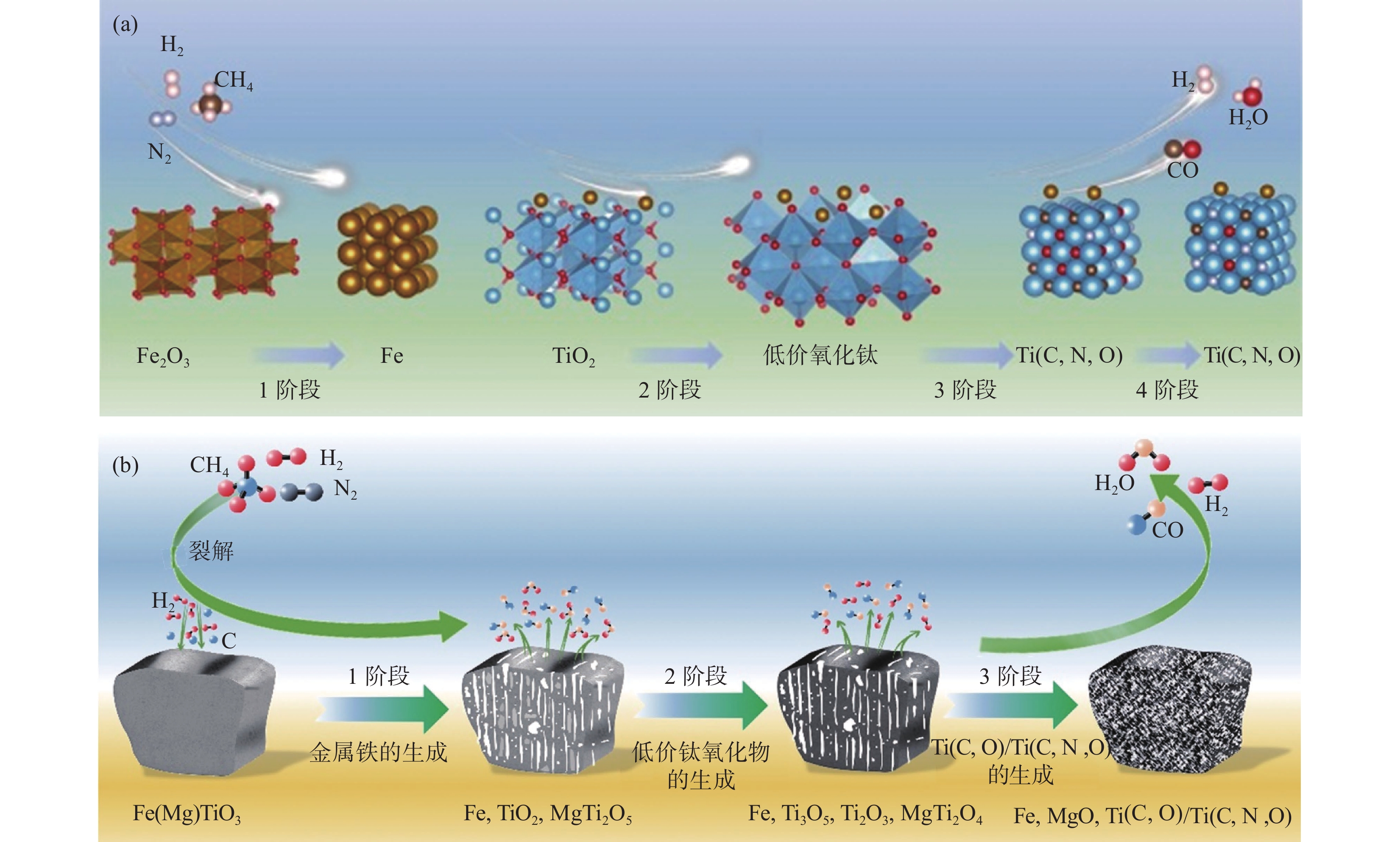
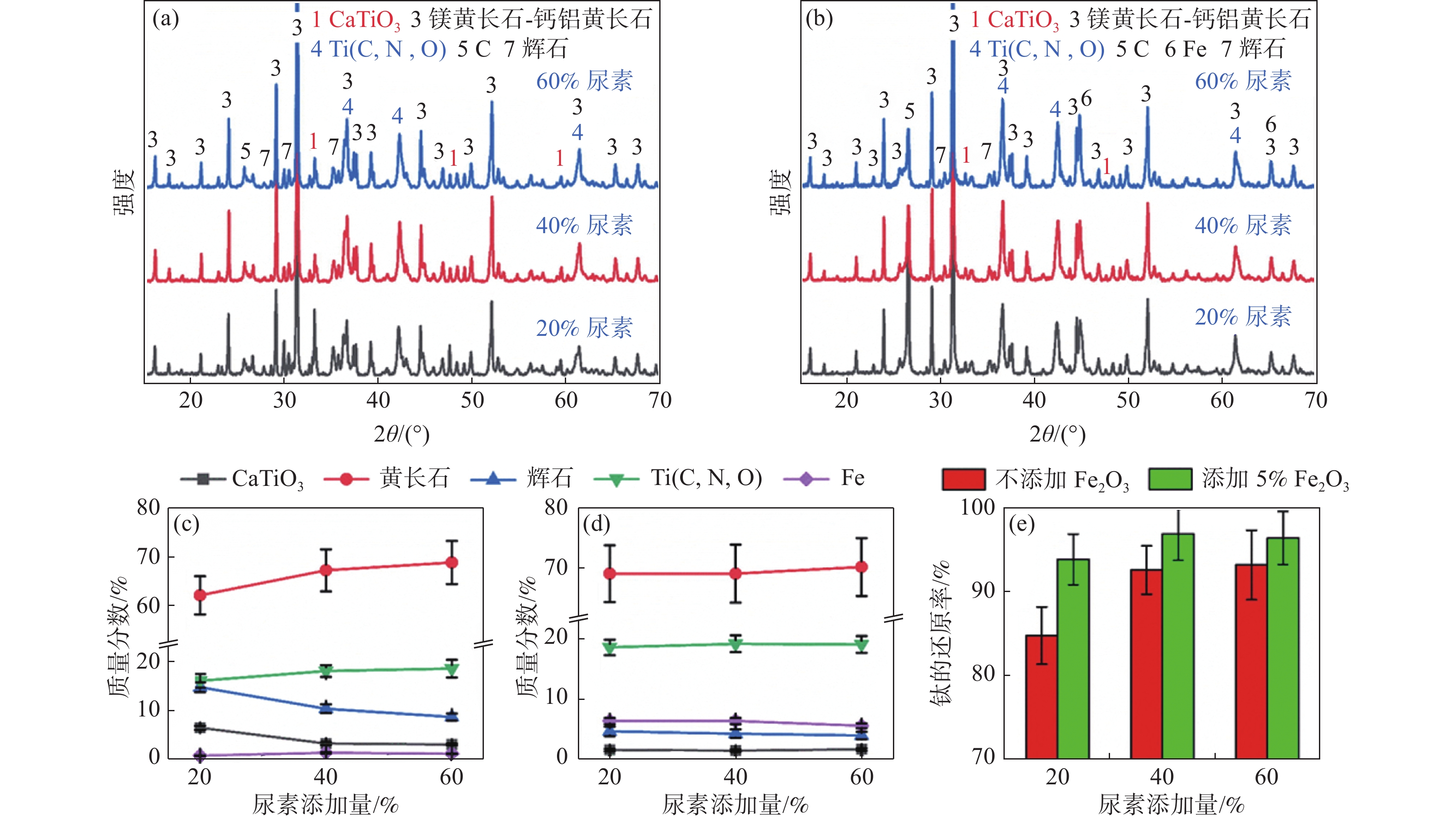
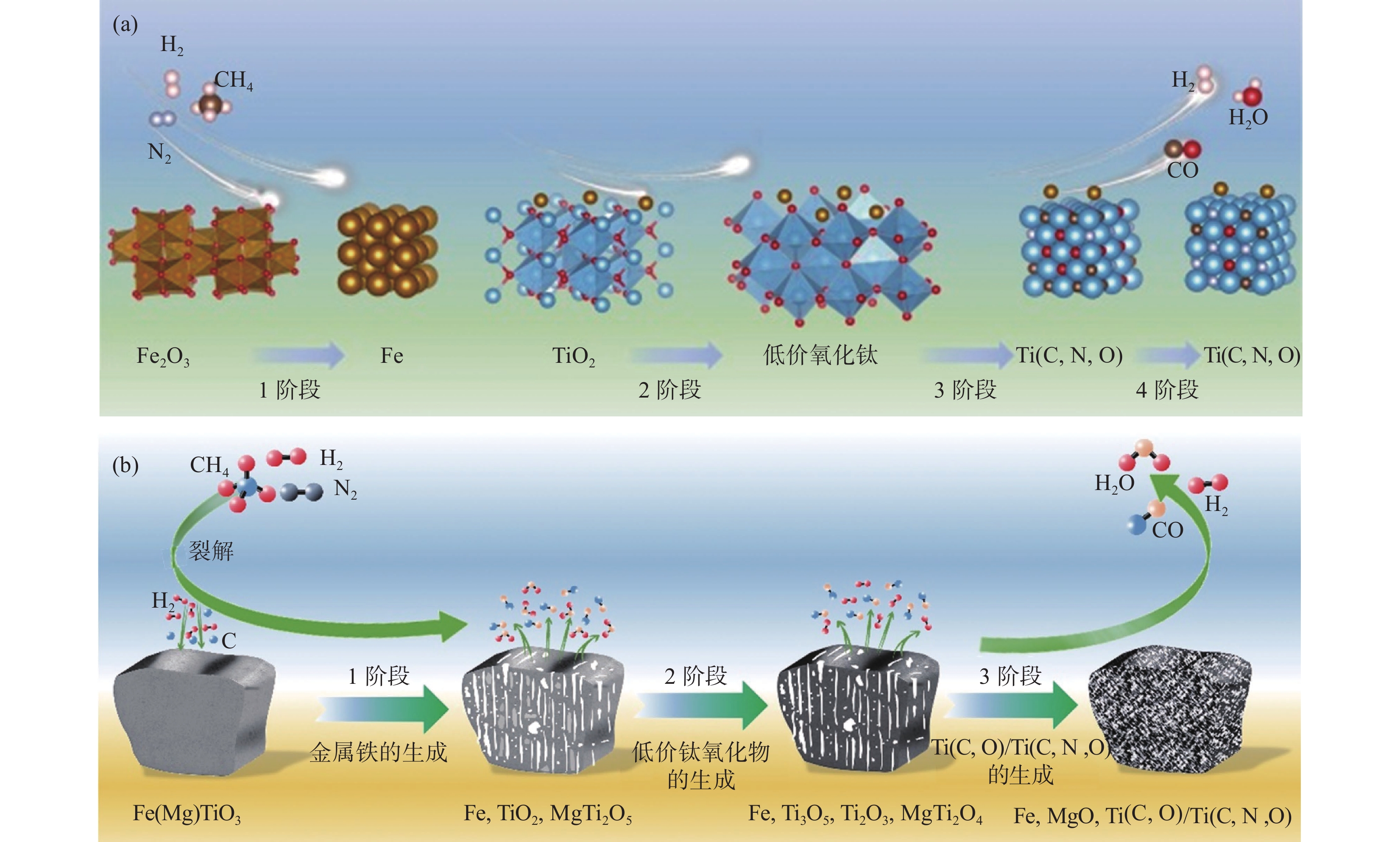
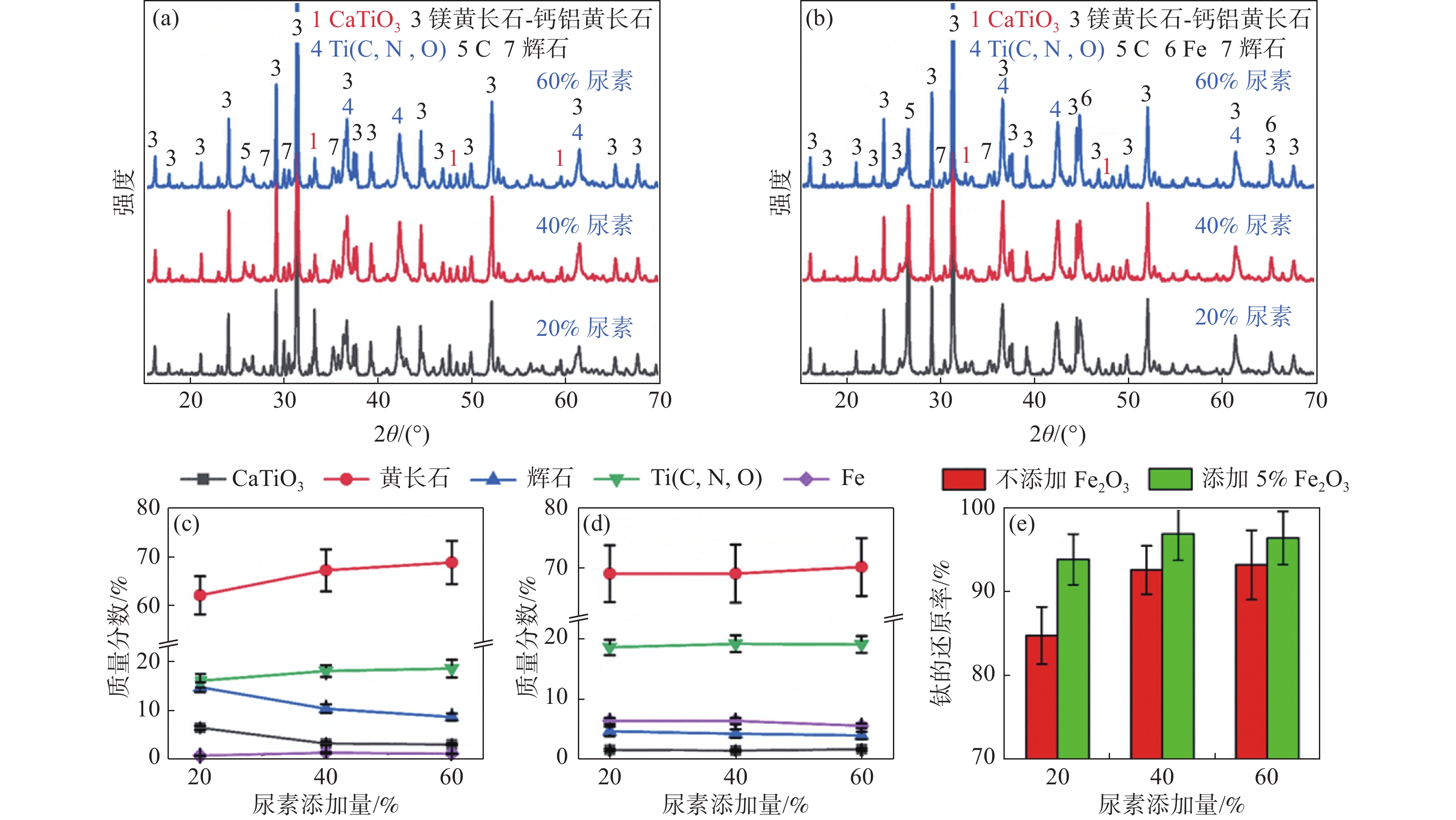
1200 ℃,减小颗粒粒径、采用预氧化处理均能改善气体的扩散传质,从而加快还原速率;不同的钛铁矿(未风化钛铁矿、风化钛铁矿和天然金红石)具有相似的还原顺序:首先是铁氧化物的还原,然后是TiO2被逐步还原为Magne'li相、Ti3O5、Ti2O3和最终的Ti(C, O)。范刚强等[26−28]的研究表明,在CH4-H2/CH4-H2-N2体系中,添加少量金属铁作为催化剂能够有效催化CH4在钛氧化物反应界面的裂解,从而促进还原速率;N2的加入能够降低金红石和钛精矿的还原温度,同时使还原产物Ti(C, O)向Ti(C, N, O)转变,还原过程示意图如图5所示。含钛高炉渣在高温下会发生烧结,阻碍还原反应的进行,添加木屑和尿素可改善其透气性,优化动力学条件,
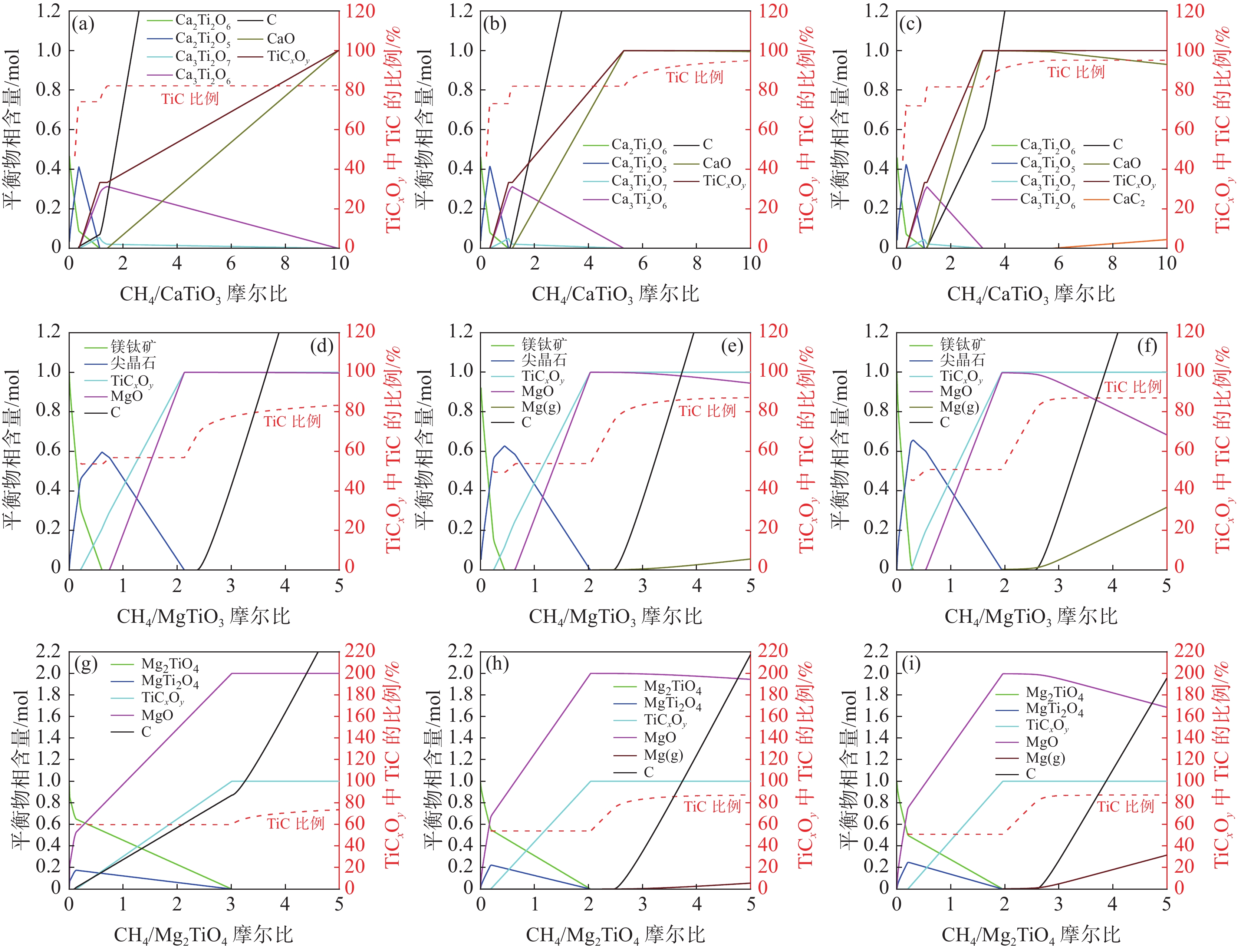
1150 ℃时便能将高炉渣中大部分含钛物相还原碳氮化(图6)。此外,张润等[10, 29−31]的理论计算表明(图7),镁钛矿和黑钛石在
1200 ℃以上被CH4-H2还原碳化,钙钛矿在1300 ℃以上被CH4-H2还原碳化,其试验结果与热力学计算结果吻合良好。温度的升高能加快含钛物相的还原碳化速率,促进还原产物Ti(C, O)的进一步脱氧,但过高的温度(>1400 ℃)会造成样品表面大量的沉积碳堆积,阻碍还原反应的进行。基于CH4-H2体系的氢还原技术,作为含钛资源还原的新途径,展现出了显著的节能与减排潜力,并有望产出诸如Ti(C, O)及Ti(C, N, O)等高价值产物。然而,该技术的实施仍面临高温下碳沉积、原料烧结以及产物分离提纯等关键挑战。因此,研究者们需要进一步深入研究和探索如何更有效地控制反应条件,如温度调控、气体组成优化、原料固体结构强化,以减轻碳沉积和原料烧结现象;同时,也需要开发高效的产物分离与提纯技术,确保生成的Ti(C, O)和Ti(C, N, O)等产物的高质量产出。
4. 基于CH4-H2体系的其他金属氧化物还原研究
4.1 镍氧化物的还原
镍是重要的战略储备金属,对经济发展具有极其重要的作用。Alizadeh等[32]用热重法研究了CH4还原多孔氧化镍球团的反应,并基于简单颗粒模型计算得到600~725 ℃范围内活化能为267 kJ/mol。但是此模型忽略了体积流动效应、固体结构变化和产物层阻力等因素对还原过程的影响。因此,Rashidi等[13, 33]也基于修正颗粒模型和随机孔隙模型对CH4还原NiO的动力学参数进行了研究,得到的反应活化能分别为258 kJ/mol和251 kJ/mol。Altay等[34]也发现,在727~
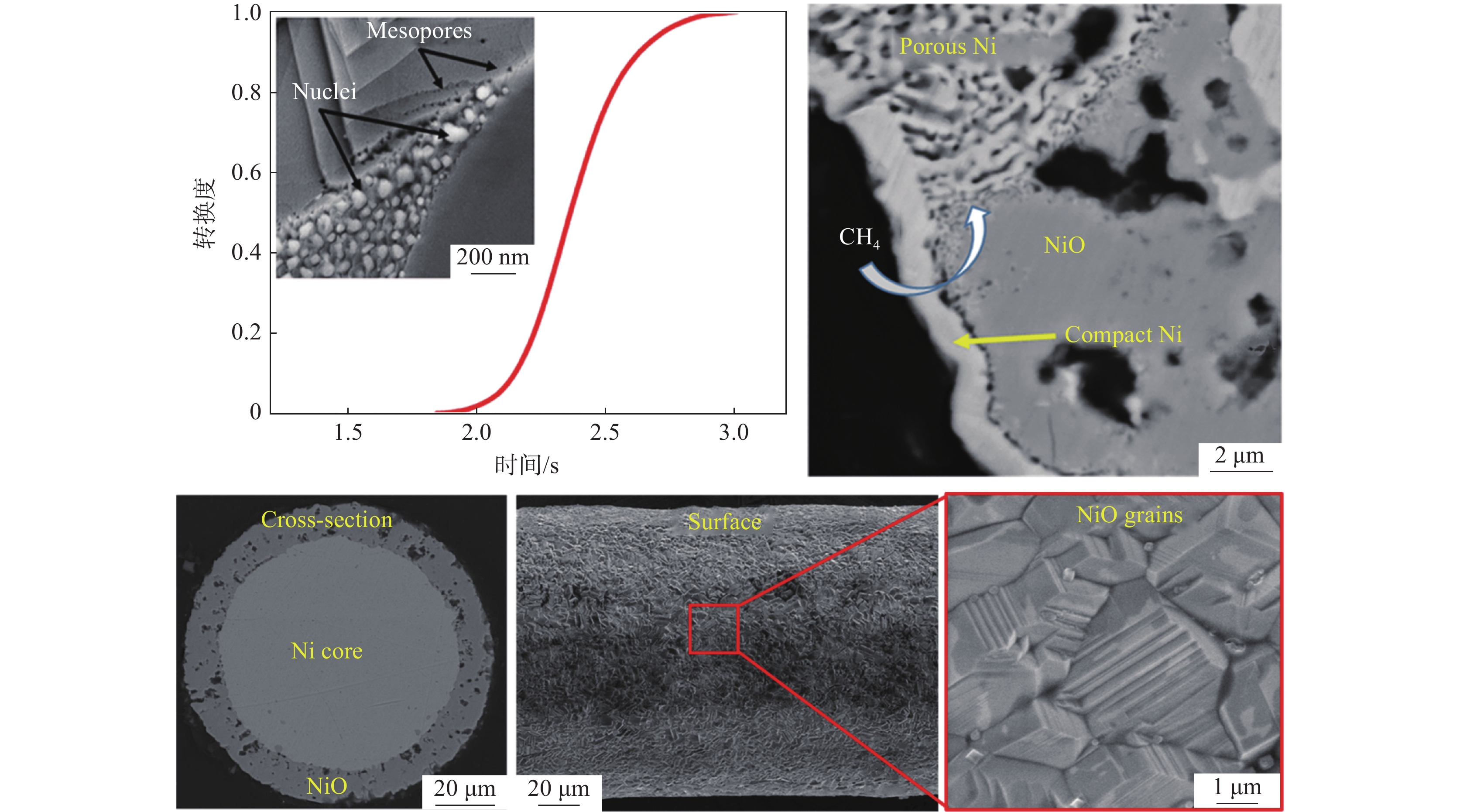
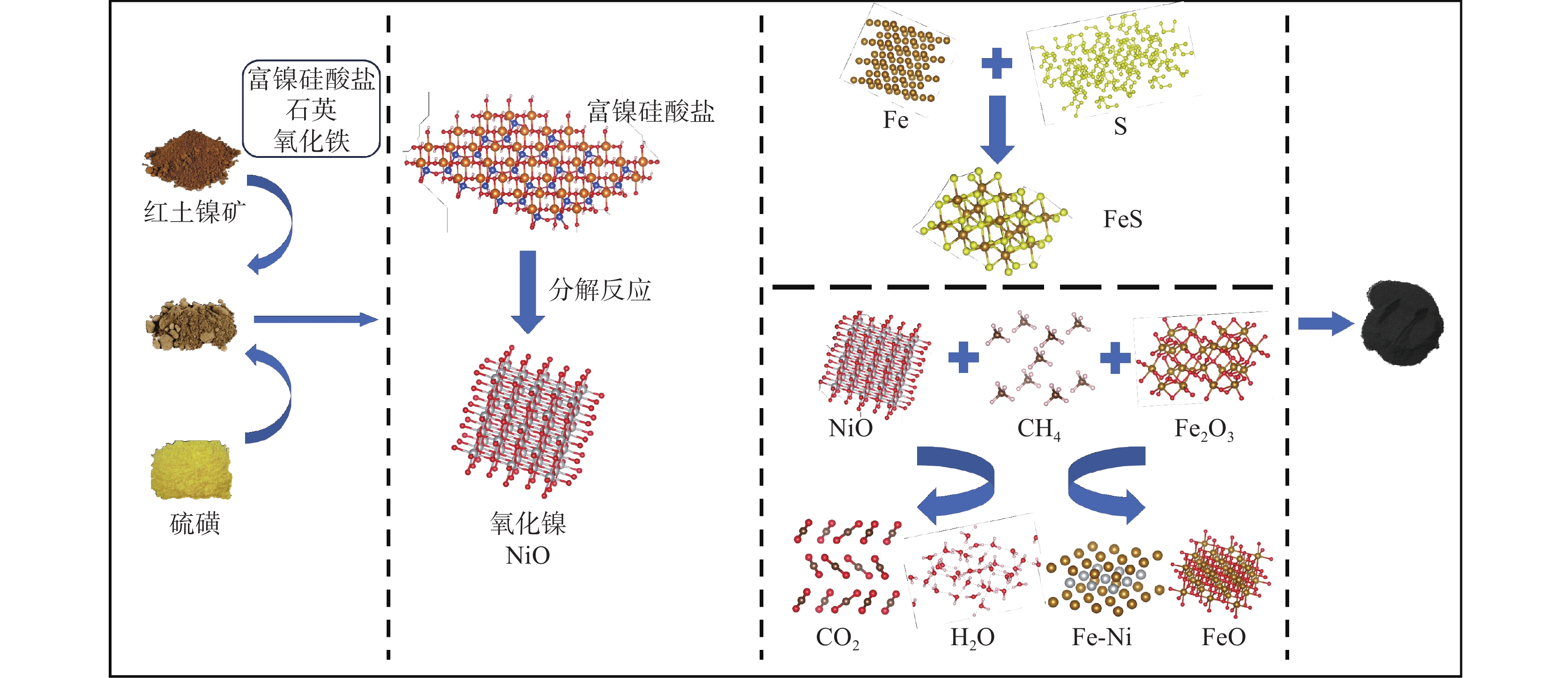
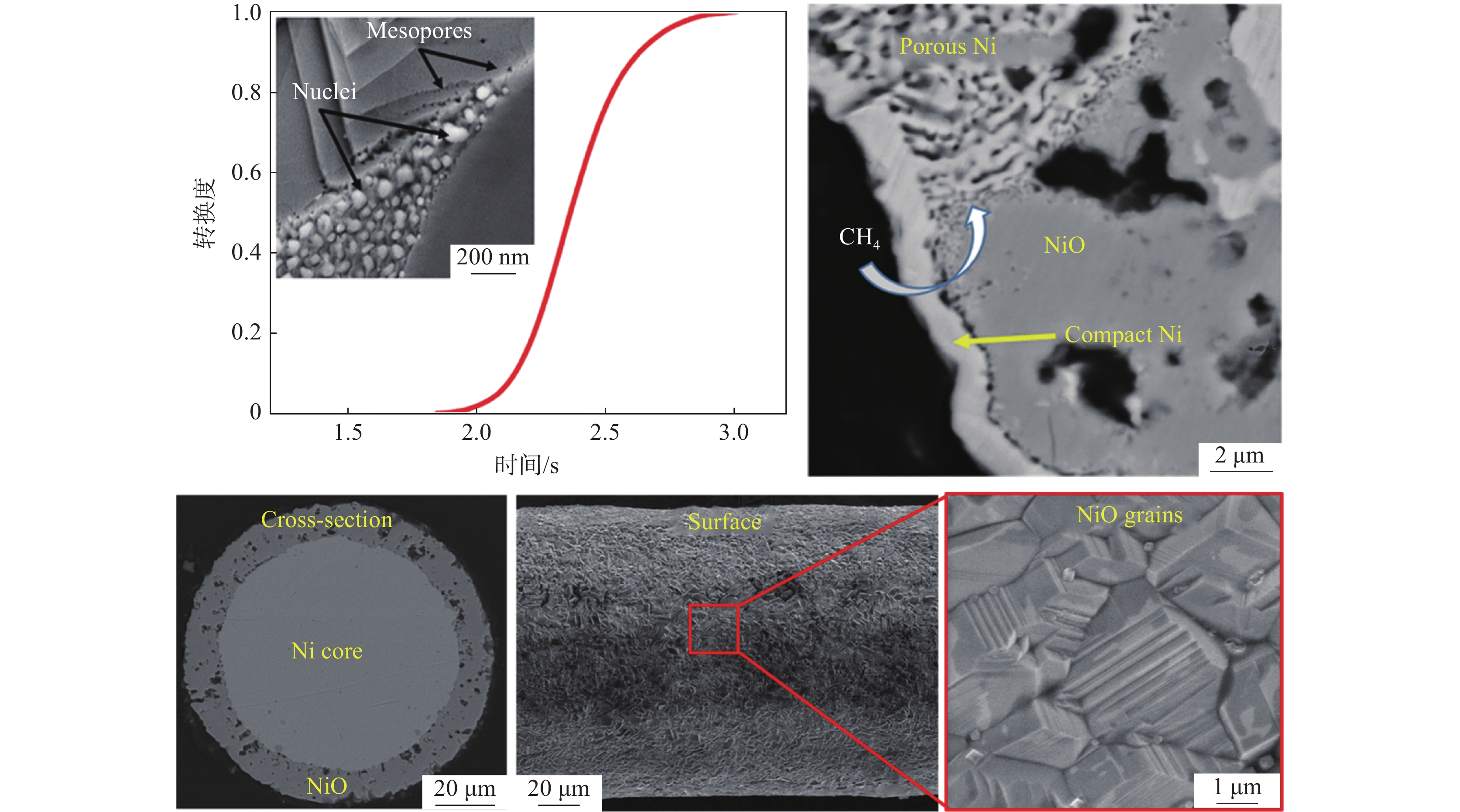
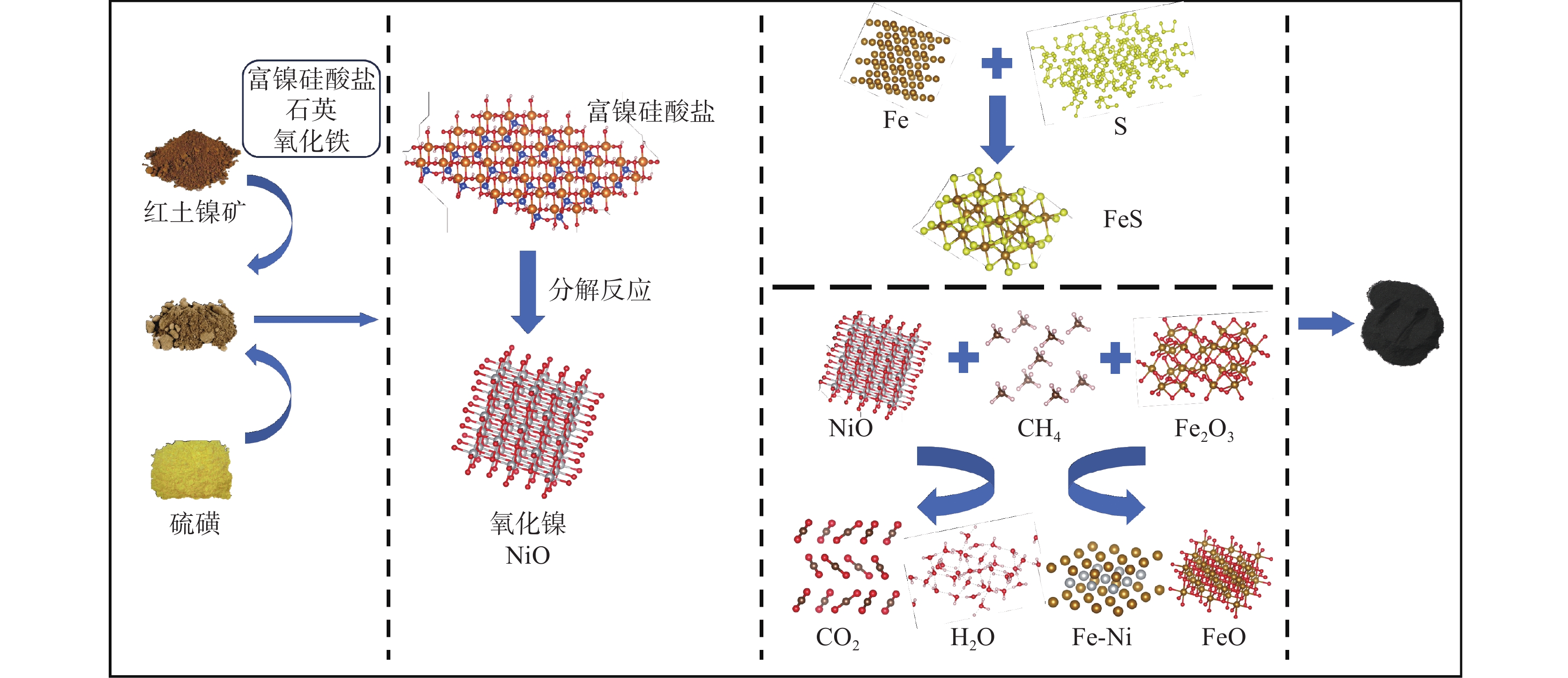
1027 ℃范围内,松散NiO粉末与纯CH4的反应基本上由两个连续的不同阶段组成:NiO的还原和预还原Ni颗粒上的C沉积。NiO的反应过程可用成核—生长和几何收缩模型来描述,化学动力学和气体外扩散机制控制总反应速率。Kharatyan等[35]采用新量热法报道了CH4对大块多晶NiO的还原过程。该方法能实现NiO/Ni导线的快速、可控加热,并能连续采集包括电功率、导线电阻率和温度在内的关键数据。结合非原位电子显微镜的观察结果,他们发现大块多晶NiO的反应动力学可以用Avrami-Erofeev成核—生长模型较好地描述:初始生成的Ni原子会吸引周围的NiO形核,成核周围形成孔洞,孔洞结构迅速成长为块状NiO,如图8所示。Pickles等[36]发现CH4在400~800 ℃对红土镍矿具有良好的还原性能。而李博等[37]的试验研究表明,红土镍矿中镍金属化率在600 ℃以下随温度的升高而增加,随着温度的进一步升高,红土镍矿中的硅酸镁会再结晶生成致密的镁橄榄石相,阻碍了镍和铁的还原,CH4裂解产生的积碳也会抑制镍的进一步还原。综合考虑镍铁金属化率,700 ℃为较佳还原温度。刘菲等[38]发现,在800 ℃、CH4浓度20%和添加4%单质硫的条件下还原60 min,红土镍矿的镍、铁金属化率分别可达98.01%和8.44%,如图9所示。单质硫的添加使得焙烧过程中生成了FeS,促进了镍铁颗粒的团聚,进一步抑制铁氧化物的还原。这一变化有利于获得更高纯度的镍产品,同时降低了铁的含量。
当前,研究者们已基本阐明了CH4还原NiO的反应机理,并对红土镍矿的还原过程进行了初步探索。CH4低温还原红土镍矿被视为未来金属镍生产的一个潜在发展方向,但仍存在一些待解问题:红土镍矿中铁与镍之间的还原分离机理尚未研究透彻,硅酸镁等杂质对还原过程的影响也未找到有效解决方案。此外,金属铁和镍均会催化CH4裂解,引发碳沉积问题,这也是CH4还原红土镍矿技术推广应用所面临的挑战。
4.2 锌氧化物的还原
锌是一种具有广泛工业用途的金属,传统的火法冶金需要在高温(约
1200 ℃)下由焦炭还原。CH4因高热力学反应性,能将氧化锌还原温度降至约850 ℃,已成为锌生产技术的新关注点。Ale Ebrahim等[39]研究了CH4对无孔ZnO球团的还原行为,并采用尖锐界面模型计算得到了840~930 ℃范围内的反应活化能为280.705 kJ/mol。此外,考虑到金属锌的高温挥发性,Ale Ebrahim等[40]还构建了考虑外传质、体积流动和表面反应影响的混合控制模型,用以预测高温(1000 ℃)下大颗粒ZnO的反应。敖先权等[41]在熔融盐体系中,通过CH4还原氧化锌成功制取了合成气和金属锌。目前,CH4还原氧化锌的研究已取得一定进展,但仍面临若干未解难题。特别是金属锌的高温挥发导致还原过程与经典的气固还原过程存在差异。在这一过程中,温度的控制以及动力学条件的优化对于提升反应效率和产物回收率至关重要。与此同时,随着锌的应用领域不断拓展,氧化锌矿等资源的开发利用正日益受到重视,CH4还原氧化锌技术将具备极大的应用前景。
4.3 钴氧化物的还原
钴金属广泛应用于工业领域,尤其在超级合金制造中不可或缺。用CH4代替C作为还原剂有助于降低钴氧化物的还原温度,减少冶炼过程中温室气体的排放。
Khoshandam等[42]研究了CH4还原多孔CoO球团的过程,发现提高CH4浓度和温度均能加快还原速率,但CH4裂解导致的碳沉积问题会阻碍还原反应的进行,流化床反应器可减轻此影响。基于简单颗粒模型,其得出800~950 ℃下CH4还原CoO的反应活化能为(155±20) kJ/mol。Shirchi等[43]以CH4为还原剂,在间歇式流化床反应器中对粉末状CoO进行了还原研究。他们基于未反应核收缩模型计算出800~950 ℃范围内的反应活化能为120 kJ/mol,并发现该过程中的化学反应是动力学限制步骤。这一发现为进一步优化CH4还原CoO的条件和机制提供了重要依据。
迄今为止,CH4还原钴氧化物的研究仍处在初级阶段,且主要聚焦于CoO的还原过程,这一领域仍存在着众多亟待解决的关键问题。其中,在高温和钴催化的双重作用下,CH4裂解导致的碳沉积问题尤为棘手。此外,对复杂含钴矿物还原机理的探究尚显不足,这无疑限制了该技术的广泛应用。
4.4 铬氧化物的还原
金属铬在不锈钢、合金制造、电镀层制备等领域具有广泛应用。铬铁矿作为铬资源的主要来源,其传统电弧炉工艺生产高碳铬铁存在能耗高、碳排放量大的问题。而采用CH4-H2-Ar气体在841 ℃以上即可使铬铁矿开始还原,这为铬的低碳生产提供了新途径[44]。
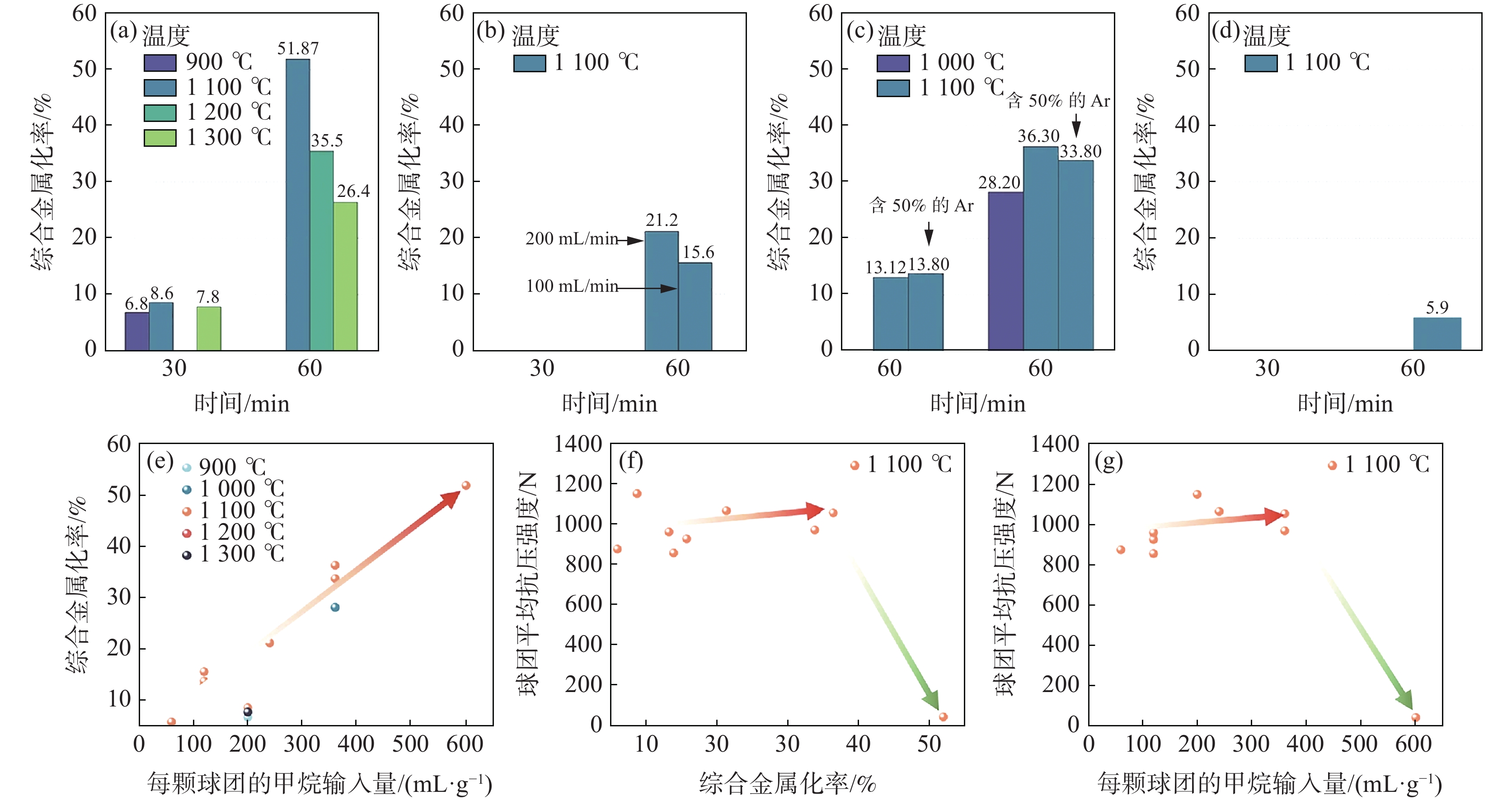
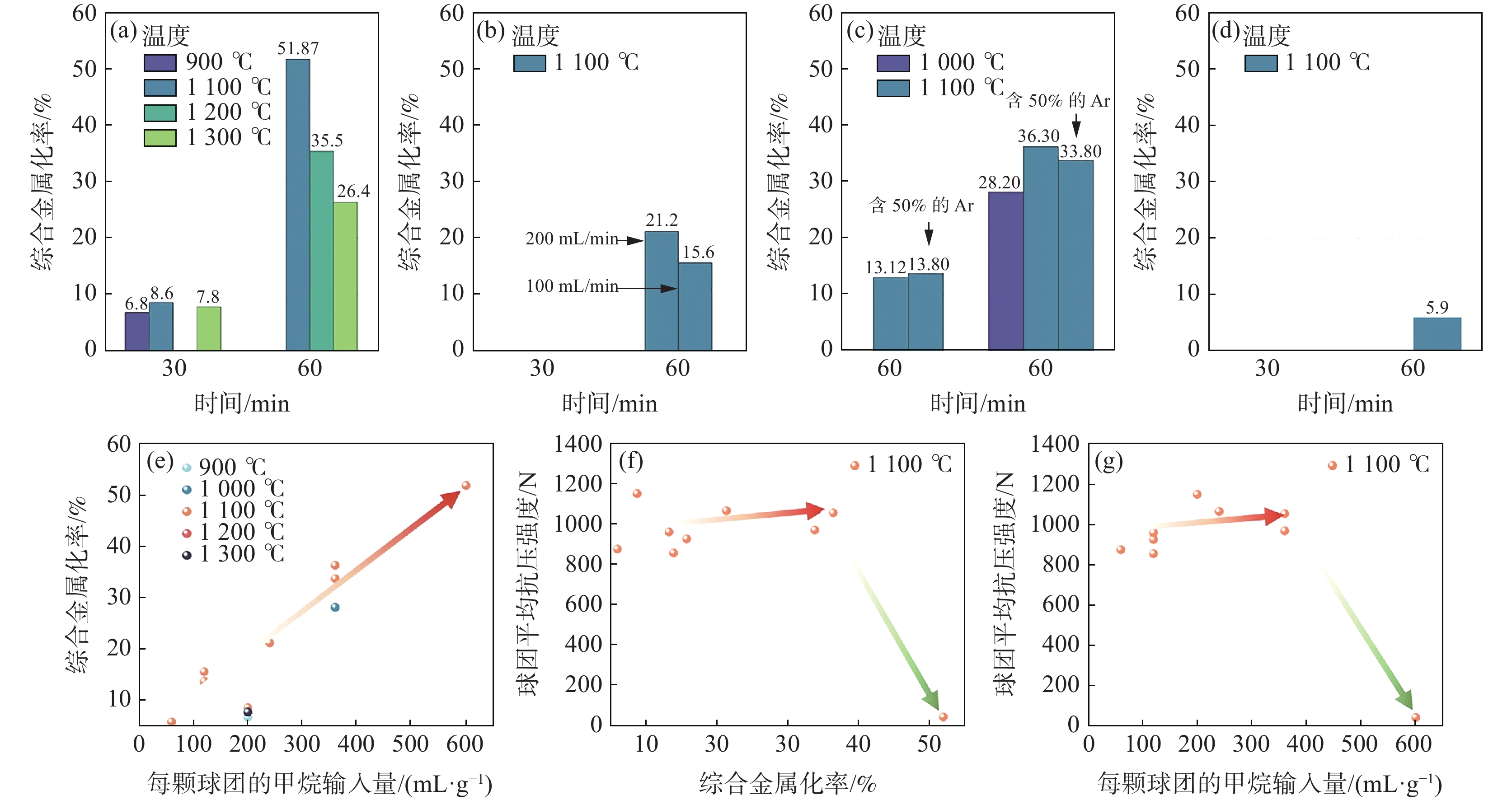
Leikola和Anacleto等[45−46]的研究均发现,CH4-H2-Ar气体对铬氧化物的还原速率远超石墨,这归因于CH4裂解产生的高活性碳(活度远大于石墨)。Wu等[47]研究了温度、时间和CH4比例对铬铁矿球团还原的具体影响(图10),发现CH4裂解产生的高活性碳有效分解和还原了铬铁矿尖晶石,而CH4的通入量成为影响球团强度的主要因素,最佳还原条件为CH4浓度10%,H2浓度90%,温度
1100 ℃,还原时间3 h。此条件下铁的金属化率为69.3%,铬的金属化率为10.3%,综合金属化率为36.3%。CH4还原铬氧化物的前景在环境科学与资源利用领域具有显著的潜力和重要性,被视为替代现有铬铁生产技术的一种潜在工艺。目前的研究已经基本探明含CH4气体还原碳化氧化铬的反应机理与反应条件。然而,对于铬铁矿等矿石的复杂反应机理还研究得不够透彻,有价金属的综合回收利用率较低,这限制了该工艺在实际生产中的应用和推广。
4.5 锰氧化物的还原
当前,氧化锰矿石的冶炼主要依赖于碳热还原法,这一过程需要在
1400 ℃以上的高温条件下进行,由此带来了高CO2排放以及锰的蒸发损失问题。为改善这一现状,相关研究者尝试使用CH4-H2混合气对锰氧化物进行还原碳化,并且已经取得了初步的成效。Anacleto和Ostrovski等[48−49]指出,MnO2可被含CH4气体还原为Mn7C3,且还原顺序为:MnO2<Mn2O3<Mn3O4<MnO<Mn7C3。在
1000 ~1200 ℃下,混合气中CH4含量需>10%、H2含量需>20%才能保证还原过程顺利进行;添加一定量的Fe3O4等添加剂可提高还原速率;气体中CO的存在会强烈地阻碍还原过程。此外,Liu等[50]研究了气体配比和温度(1100 ~1250 ℃)对MnO2还原的影响,发现CH4/(CH4+H2)摩尔比为0.2时,MnO2的还原程度随温度的升高而增大;然而,由于沉积碳的形成,即使提高温度和延长时间,MnO向Mn7C3的深度还原也会受到阻碍。因此,为了调控沉积碳的产生,需要精细调节气体混合物中CH4/(CH4+H2)的摩尔比。目前CH4-H2还原锰氧化物的研究已经取得了一定的进展,研究者们对于多价态锰氧化物的还原规律和还原过程中的物相和微观结构演变过程都进行了深入探讨。通过不断的试验和优化,研究人员已经探索出多种有效的还原方法和条件,为提高锰资源的利用率和降低生产成本提供了有力的支持。
5. 总结与展望
热力学与试验研究均已表明,CH4气体拥有比煤炭和氢气更优异的还原能力。与火法冶金领域传统的“碳冶金”方法相比,基于CH4-H2体系的氢还原技术可实现金属矿物的低碳、低温、低能耗还原,能够从源头减少金属冶炼的碳排放。丰富的页岩气等天然氢能资源以及工业制氢技术的蓬勃发展使得基于CH4-H2体系的氢还原技术在氧化物还原领域具有广阔的应用前景和重要的研究价值。
目前,研究者们对CH4还原的热力学和动力学也进行了大量的探讨,已经明确了大部分金属氧化物的还原条件。然而,目前有关CH4还原复杂金属矿物的研究还比较少,高温下的积碳、样品烧结等问题还尚未解决,距离工业化应用仍有距离。未来,我们需要继续深入研究该技术的反应机理和影响因素,优化试验方案和操作条件,以实现更高效、更环保的氧化物还原过程,为相关领域的可持续发展做出贡献。
-
-
[1] Zhang Jianliang, Zong Yanbing, Li Kejiang, et al. Progress and outlook of new low-carbon ironmaking technologies in the world[J]. Iron and Steel, 2024, 59(9): 45-55, 155. (张建良, 宗燕兵, 李克江, 等. 全球低碳炼铁新工艺技术进展及展望[J]. 钢铁, 2024, 59(9): 45-55, 155.Zhang Jianliang, Zong Yanbing, Li Kejiang, et al. Progress and outlook of new low-carbon ironmaking technologies in the world[J]. Iron and Steel, 2024, 59(9): 45-55, 155. [2] Tang Jue, Chu Mansheng, Li Feng, et al. Development and progress on hydrogen metallurgy[J]. International Journal of Minerals Metallurgy and Materials, 2020,27(6):713-723. doi: 10.1007/s12613-020-2021-4 [3] Jiang Zhouhua, Yang Ce, Zhu Hongchun, et al. Research status and prospect of hydrogen metallurgy steelmaking technology[J]. Iron & Steel, 2024,59(9):140-155. (姜周华, 杨策, 朱红春, 等. 氢冶金炼钢技术的研究现状与展望[J]. 钢铁, 2024,59(9):140-155.Jiang Zhouhua, Yang Ce, Zhu Hongchun, et al. Research status and prospect of hydrogen metallurgy steelmaking technology[J]. Iron & Steel, 2024, 59(9): 140-155. [4] Luo Dayong. Natural gas production in China from 2018-2023[J]. International Petroleum Economics, 2024,32(4):105. (罗大勇. 2018-2023年中国天然气产量[J]. 国际石油经济, 2024,32(4):105. doi: 10.3969/j.issn.1004-7298.2024.04.016Luo Dayong. Natural gas production in China from 2018-2023[J]. International Petroleum Economics, 2024, 32(4): 105. doi: 10.3969/j.issn.1004-7298.2024.04.016 [5] Bost N, Ammar M R, Bouchetou M L, et al. The catalytic effect of iron oxides on the formation of nano-carbon by the Boudouard reaction in refractories[J]. Journal of the European Ceramic Society, 2016,36(8):2133-2142. doi: 10.1016/j.jeurceramsoc.2016.02.052 [6] Shen Fengman, Ding Zhimin, Wang Shuo, et al. Development and application of carbon deposition state diagram for H-C-O system[J]. Iron and Steel, 2024,59(9):122-129. (沈峰满, 丁智敏, 王硕, 等. 关于H-C-O体系析碳状态分布图的开发与应用[J]. 钢铁, 2024,59(9):122-129.Shen Fengman, Ding Zhimin, Wang Shuo, et al. Development and application of carbon deposition state diagram for H-C-O system[J]. Iron and Steel, 2024, 59(9): 122-129. [7] Shen Fengman. Development of H-C-O system mass balance and chemical equilibrium diagram[J]. Iron and Steel, 2023,58(6):12-17. (沈峰满. H-C-O体系质量及化学平衡衡算图的开发[J]. 钢铁, 2023,58(6):12-17.Shen Fengman. Development of H-C-O system mass balance and chemical equilibrium diagram[J]. Iron and Steel, 2023, 58(6): 12-17. [8] Shen Fengman, Zhang Weiling, Zheng Aijun, et al. Regulation of carbon deposition during preparation process of hydrogen-rich reducing gas by natural gas reforming-An application example of H-C-O system mass balance and chemical equilibrium diagram[J]. Iron and Steel, 2023,58(7):9-16. (沈峰满, 章苇玲, 郑艾军, 等. 关于天然气重整制备富氢还原气体过程中析碳问题的调控——H-C-O体系质量及化学平衡衡算图的应用例[J]. 钢铁, 2023,58(7):9-16.Shen Fengman, Zhang Weiling, Zheng Aijun, et al. Regulation of carbon deposition during preparation process of hydrogen-rich reducing gas by natural gas reforming-An application example of H-C-O system mass balance and chemical equilibrium diagram[J]. Iron and Steel, 2023, 58(7): 9-16. [9] Shen Fengman, Zheng Aijun, Zheng Haiyan, et al. Thoughts on preparation of hydrogen-based reduction gas and process of direct reduction iron[J]. Iron and Steel, 2022,57(3):10-15. (沈峰满, 郑艾军, 郑海燕, 等. 关于直接还原铁工艺及还原气制备的若干思考[J]. 钢铁, 2022,57(3):10-15.Shen Fengman, Zheng Aijun, Zheng Haiyan, et al. Thoughts on preparation of hydrogen-based reduction gas and process of direct reduction iron[J]. Iron and Steel, 2022, 57(3): 10-15. [10] Zhang Run, Liu Dong, Fan Gangqiang, et al. Thermodynamic and experimental study on the reduction and carbonization of TiO2 through gas-solid reaction[J]. International Journal of Energy Research, 2019,43(9):4253-4263. doi: 10.1002/er.4551 [11] Halli P, Taskinen P, Eriҫ R H. Mechanisms and kinetics of solid state reduction of titano magnetite ore with methane[J]. Journal of Sustainable Metallurgy, 2017,3(2):191-206. doi: 10.1007/s40831-016-0063-7 [12] Lü Zepeng, Dang Jie. Mathematical modeling of the reaction of metal oxides with methane[J]. RSC Advances, 2020,10(19):11233-11243. doi: 10.1039/C9RA09418K [13] Rashidi H, Ebrahim H A, Dabir B. Reduction kinetics of nickel oxide by methane as reducing agent based on thermogravimetry[J]. Thermochimica Acta, 2013,561:41-48. doi: 10.1016/j.tca.2013.03.014 [14] Ni Hongwei, Cang Daqiang, Jiang Junpu, et al. Suitable gas-phase compositions for the preparation of iron carbide from H2-CH4 gas[J]. Journal of East China Institute of Metallurgy, 1997,3:203-208. (倪红卫, 苍大强, 姜钧普, 等. 用H2-CH4气制备碳化铁的合适气相成分[J]. 华东冶金学院学报, 1997,3:203-208.Ni Hongwei, Cang Daqiang, Jiang Junpu, et al. Suitable gas-phase compositions for the preparation of iron carbide from H2-CH4 gas[J]. Journal of East China Institute of Metallurgy, 1997, 3: 203-208. [15] Ma Jianghua, Li Guangqiang. Influence of iron ore porosity on its reduction and iron carbide generation[J]. Journal of Process Engineering, 2007,6:1132-1137. (马江华, 李光强. 铁矿石孔隙度对其还原和碳化铁生成的影响[J]. 过程工程学报, 2007,6:1132-1137. doi: 10.3321/j.issn:1009-606x.2007.06.014Ma Jianghua, Li Guangqiang. Influence of iron ore porosity on its reduction and iron carbide generation[J]. Journal of Process Engineering, 2007, 6: 1132-1137. doi: 10.3321/j.issn:1009-606x.2007.06.014 [16] Li Guangqiang, Wang Henghui, Yang Jian, et al. Preparation of iron carbide from high phosphorus oolitic hematite[J]. Advanced Materials Research, 2014,88-883:98-101. [17] Wang Henghui, Li Guangqiang, Yang Jian, et al. The behavior of phosphorus during reduction and carburization of high-phosphorus oolitic hematite with H2 and CH4[J]. Metall Mater Trans B, 2016,47:2571-2581. doi: 10.1007/s11663-016-0709-7 [18] Zhang Run, Wang Chao, You Yang, et al. Reduction and carbonization of iron concentrate with hydrogen-rich gas[J]. The Minerals, Metals & Materials Series, 2024: 29-38. [19] Ghosh D, Roy A K, Ghosh A. Reduction of ferric oxide pellets with methane[J]. Transactions of the Iron and Steel Institute of Japan, 1986,26(3):186-193. doi: 10.2355/isijinternational1966.26.186 [20] Monazam E R, Breault R W, Siriwardane R, et al. Kinetics of the reduction of hematite (Fe2O3) by methane (CH4) during chemical looping combustion: A global mechanism[J]. Chemical Engineering Journal, 2013,232:478-487. doi: 10.1016/j.cej.2013.07.091 [21] Zhang G Q, Ostrovski O. Reduction of ilmenite concentrates by methane-containing gas: Part I. Effects of ilmenite composition, temperature and gas composition[J]. Canadian Metallurgical Quarterly, 2001,40(3):317-326. doi: 10.1179/cmq.2001.40.3.317 [22] Zhang Guangqing, Ostrovski O. Reduction of ilmenite concentrates by methane containing gas, Part II: Effects of preoxidation and sintering[J]. Canadian Metallurgical Quarterly, 2001,40(4):489-497. doi: 10.1179/cmq.2001.40.4.489 [23] Zhang Guangqing, Ostrovski O. Kinetic modeling of titania reduction by a methane-hydrogen-argon gas mixture[J]. Metallurgical and Materials Transactions B, 2001,32(3):465-473. doi: 10.1007/s11663-001-0032-8 [24] Dang Jie, Fatollahi F F, Pistorius P C, et al. Synthesis of titanium oxycarbide from titanium slag by methane-containing gas[J]. Metallurgical and Materials Transactions B, 2018,49(1):123-131. doi: 10.1007/s11663-017-1123-5 [25] Dang Jie, Fatollahi F F, Pistorius P C, et al. Synthesis of titanium oxycarbide from concentrates of natural ilmenite (weathered and unweathered) and natural rutile, using a methane-hydrogen gas mixture[J]. Metallurgical and Materials Transactions B, 2017,48(5):2440-2446. doi: 10.1007/s11663-017-1048-z [26] Fan Gangqiang, Hou Youling, Huang Dejun, et al. Synthesis of Ti(C, N, O) ceramic from rutile at low temperature by CH4-H2-N2 gas mixture[J]. International Journal of Refractory Metals and Hard Materials, 2021,101:105659. doi: 10.1016/j.ijrmhm.2021.105659 [27] Fan Gangqiang, Dang Jie, Zhang Run, et al. Synthesis of Ti(C, O, N) from ilmenite at low temperature by a novel reducing and carbonitriding approach[J]. International Journal of Energy Research, 2020,44(6):4861-4874. doi: 10.1002/er.5283 [28] Fan Gangqiang, Wang Meng, Dang Jie, et al. A novel recycling approach for efficient extraction of titanium from high-titanium-bearing blast furnace slag[J]. Waste Management, 2021,120:626-634. doi: 10.1016/j.wasman.2020.10.024 [29] Zhang Run, Dang Jie, Liu Dong, et al. Reduction of perovskite-geikielite by methane-hydrogen gas mixture: Thermodynamic analysis and experimental results [J]. Science of The Total Environment, 2020,699: 134355. [30] Zhang Run, Fan Gangqiang, Song Mingbo, et al. Thermodynamic analysis and reduction of anosovite with methane at low temperature[C]//Energy Technology 2020: Recycling, Carbon Dioxide Management, and Other Technologies. Springer International Publishing, 2020:285-294. [31] Zhang Run, Hou Youling, Fan Gangqiang, et al. Gas-based reduction and carbonization of titanium minerals in titanium-bearing blast furnace slag: A combined thermodynamic, experimental and DFT study[J]. International Journal of Hydrogen Energy, 2022,47(12):7586-7599. doi: 10.1016/j.ijhydene.2021.12.119 [32] Alizadeh R, Jamshidi E, Ale Ebrahim H. Kinetic study of nickel oxide reduction by methane[J]. Chemical Engineering & Technology, 2007,30(8):1123-1128. [33] Rashidi H, Ale E H, Dabir B. Application of random pore model for synthesis gas production by nickel oxide reduction with methane[J]. Energy Conversion and Management, 2013,74:249-260. doi: 10.1016/j.enconman.2013.04.044 [34] Altay M C, Eroglu S. Isothermal reaction of NiO powder with undiluted CH4 at 1000 K to 1300 K (727 °C to 1027 °C)[J]. Metallurgical and Materials Transactions B, 2017,48(4):2067-2076. doi: 10.1007/s11663-017-0991-z [35] Kharatyan S L, Chatilyan H A, Manukyan K V. Kinetics and mechanism of nickel oxide reduction by methane[J]. The Journal of Physical Chemistry C, 2019,123(35):21513-21521. doi: 10.1021/acs.jpcc.9b04506 [36] Pickles C A, Anthony W. Thermodynamic modelling of the reduction of a saprolitic laterite ore by methane[J]. Minerals Engineering, 2018,120:47-59. doi: 10.1016/j.mineng.2018.02.006 [37] Li Bo, Ding Zhiguang, Wei Yonggang, et al. Reduction of nickel and iron from low-grade nickel laterite ore via a solid-state deoxidization method using methane[J]. Materials Transactions, 2018,59(7):1180-1185. doi: 10.2320/matertrans.M2017351 [38] Liu Fei, Li Bo, Wei Yonggang, et al. Effect of elemental sulfur on the reduction process of laterite nickel ore under the action of methane[J]. Materials Transactions, 2023,64(12):2754-2763. doi: 10.2320/matertrans.MT-M2022189 [39] Ale E H, Jamshidi E. Kinetic study of zinc oxide reduction by methane[J]. Chemical Engineering Research & Design, 2001,79(A1):62-70. [40] Ale E H, Jamshidi E. Effect of mass transfer and bulk flow on the zinc oxide reduction by methane[J]. Industrial & Engineering Chemistry Research, 2002,41(11):2630-2636. [41] Ao Xianquan. Basic research on the synthesis of syngas and zinc metal by methane reduction of zinc oxide in molten salt system[D]. Kunming: Kunming University of Science and Technology, 2008. (敖先权. 熔盐体系中甲烷还原氧化锌制取合成气和金属锌的基础研究[D]. 昆明: 昆明理工大学, 2008.Ao Xianquan. Basic research on the synthesis of syngas and zinc metal by methane reduction of zinc oxide in molten salt system[D]. Kunming: Kunming University of Science and Technology, 2008. [42] Khoshandam B, Jamshidi E, Kumar R V. Reduction of cobalt oxide with methane[J]. Metallurgical and Materials Transactions B, 2004,35(5):825-828. doi: 10.1007/s11663-004-0076-7 [43] Shirchi S, Khoshandam B, Hormozi F. Reduction kinetics of cobalt oxide powder by methane in a fluidized bed reactor[J]. Journal of the Taiwan Institute of Chemical Engineers, 2015,51:171-176. doi: 10.1016/j.jtice.2015.01.030 [44] Qayyum M A, Reeve D A. Reduction of chromites to sponge ferrochromium in methane-hydrogen mixtures[J]. Canadian Metallurgical Quarterly, 1976,15(3):193-200. doi: 10.1179/cmq.1976.15.3.193 [45] Leikola M, Taskinen P, Eric R H. Reduction of Kemi chromite with methane[J]. Journal of the Southern African Institute of Mining and Metallurgy, 2018,118(6):575-580. [46] Anacleto N, Ostrovski O. Solid-state reduction of chromium oxide by methane-containing gas[J]. Metallurgical and Materials Transactions B, 2004,35(4):609-615. doi: 10.1007/s11663-004-0001-0 [47] Wu Shaowen, Feng Xiaoming, Zhang Yanling, et al. Methane-hydrogen-based pre-reduction chromite: reduction behavior and pellet compressive strength[J]. Journal of Metals, 2024,76(9):4858-4872. [48] Anacleto N, Ostrovski O, Ganguly S. Reduction of manganese oxides by methane-containing gas[J]. ISIJ International, 2004,44(9):1480-1487. doi: 10.2355/isijinternational.44.1480 [49] Ostrovski O, Zhang G. Reduction and carburization of metal oxides by methane-containing gas[J]. AIChE Journal, 2005,52(1):300-310. [50] Liu Bingbing, Zhang Yuanbo, Su Zijian, et al. Thermodynamic analysis and reduction of MnO2 by methane-hydrogen gas mixture[J]. Journal of Metals, 2017,69(9):1669-1675. -






 下载:
下载:










 下载:
下载: